Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells
- PMID: 11909953
- PMCID: PMC133714
- DOI: 10.1128/MCB.22.8.2586-2597.2002
Cripto-1 activates nodal- and ALK4-dependent and -independent signaling pathways in mammary epithelial Cells
Abstract
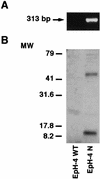
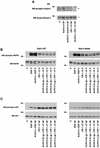
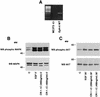
Cripto-1 (CR-1), an epidermal growth factor-CFC (EGF-CFC) family member, has a demonstrated role in embryogenesis and mammary gland development and is overexpressed in several human tumors. Recently, EGF-CFC proteins were implicated as essential signaling cofactors for Nodal, a transforming growth factor beta family member whose expression has previously been defined as embryo specific. To identify a receptor for CR-1, a human brain cDNA phage display library was screened using CR-1 protein as bait. Phage inserts with identity to ALK4, a type I serine/threonine kinase receptor for Activin, were identified. CR-1 binds to cell surface ALK4 expressed on mammalian epithelial cells in fluorescence-activated cell sorter analysis, as well as by coimmunoprecipitation. Nodal is coexpressed with mouse Cr-1 in the mammary gland, and CR-1 can phosphorylate the transcription factor Smad-2 in EpH-4 mammary epithelial cells only in the presence of Nodal and ALK4. In contrast, CR-1 stimulation of mitogen-activated protein kinase and AKT in these cells is independent of Nodal and ALK4, suggesting that CR-1 may modulate different signaling pathways to mediate its different functional roles.
Figures
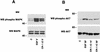


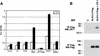


References
-
- Attisano, L., C. Silvestri, L. Izzi, and E. Labbe. 2001. The transcriptional role of Smads and FAST (FoxH1) in TGFβ and activin signaling. Mol. Cell. Endocrinol. 180:3-11. - PubMed
-
- Bamford, R. N., E. Rossler, R. D. Burdine, U. Saplakoglu, J. dela Cruz, M. Splitt, J. Towbin, P. Bowers, B. Marino, A. F. Schier, M. M. Shen, M. M. Muenke, and B. Casey. 2000. Loss of function mutations in the EGF-CFC gene CFC1 are associated with human left-right laterality defects. Nat. Genet. 26:365-369. - PubMed
-
- Bianco, C., S. Kannan, M. De Santis, M. Seno, C. K. Tang, I. Martinez-Lacaci, N. Kim, B. Wallace-Jones, M. E. Lippman, A. Ebert, C. Wechselberger, and D. S. Salomon. 1999. Cripto-1 indirectly stimulates the tyrosine phosphorylation of erb B-4 through a novel receptor. J. Biol. Chem. 274:8624-8629. - PubMed
-
- Brandt, R., N. Normanno, W. J. Gullick, J. H. Lin, R. Harkins, D. Schneider, B. Jones, F. Ciardiello, M. G. Persico, F. Armenante, N. Kim, and D. S. Salomon. 1994. Identification and biological characterization of an epidermal growth factor-related protein: Cripto-1. J. Biol. Chem. 269:17320-17328. - PubMed
-
- Brennan, J., C. C. Lu, D. P. Norris, T. A. Rodriguez, R. S. Beddington, and E. J. Robertson. 2001. Nodal signalling in the epiblast patterns the early mouse embryo. Nature 411:965-969. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
