Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux
- PMID: 11909955
- PMCID: PMC133709
- DOI: 10.1128/MCB.22.8.2607-2619.2002
Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux
Abstract
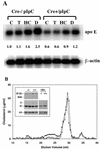
Disruption of the peroxisome proliferator-activated receptor gamma (PPAR gamma) gene causes embryonic lethality due to placental dysfunction. To circumvent this, a PPAR gamma conditional gene knockout mouse was produced by using the Cre-loxP system. The targeted allele, containing loxP sites flanking exon 2 of the PPAR gamma gene, was crossed into a transgenic mouse line expressing Cre recombinase under the control of the alpha/beta interferon-inducible (MX) promoter. Induction of the MX promoter by pIpC resulted in nearly complete deletion of the targeted exon, a corresponding loss of full-length PPAR gamma mRNA transcript and protein, and marked reductions in basal and troglitazone-stimulated expression of the genes encoding lipoprotein lipase, CD36, LXR alpha, and ABCG1 in thioglycolate-elicited peritoneal macrophages. Reductions in the basal levels of apolipoprotein E (apoE) mRNA in macrophages and apoE protein in total plasma and high-density lipoprotein (HDL) were also observed in pIpC-treated PPAR gamma-MXCre(+) mice. Basal cholesterol efflux from cholesterol-loaded macrophages to HDL was significantly reduced after disruption of the PPAR gamma gene. Troglitazone selectively inhibited ABCA1 expression (while rosiglitazone, ciglitazone, and pioglitazone had little effect) and cholesterol efflux in both PPAR gamma-deficient and control macrophages, indicating that this drug can exert paradoxical effects on cholesterol homeostasis that are independent of PPAR gamma. Together, these data indicate that PPAR gamma plays a critical role in the regulation of cholesterol homeostasis by controlling the expression of a network of genes that mediate cholesterol efflux from cells and its transport in plasma.
Figures








References
-
- Babaev, V. R., M. B. Patel, C. F. Semenkovich, S. Fazio, and M. F. Linton. 2000. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J. Biol. Chem. 275:26293-26299. - PubMed
-
- Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPARγ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. - PubMed
-
- Basheeruddin, K., C. Rechtoris, and T. Mazzone. 1992. Transcriptional and post-transcriptional control of apolipoprotein E gene expression in differentiating human monocytes. J. Biol. Chem. 267:1219-1224. - PubMed
-
- Basu, S. K., J. L. Goldstein, and M. S. Brown. 1983. Independent pathways for secretion of cholesterol and apolipoprotein E by macrophages. Science 219:871-873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
