Inducer-specific enhanceosome formation controls tumor necrosis factor alpha gene expression in T lymphocytes
- PMID: 11909956
- PMCID: PMC133734
- DOI: 10.1128/MCB.22.8.2620-2631.2002
Inducer-specific enhanceosome formation controls tumor necrosis factor alpha gene expression in T lymphocytes
Abstract
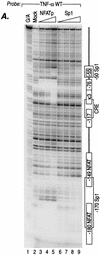
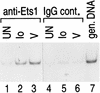
We present evidence that the inducer-specific regulation of the human tumor necrosis factor alpha (TNF-alpha) gene in T cells involves the assembly of distinct higher-order transcription enhancer complexes (enhanceosomes), which is dependent upon inducer-specific helical phasing relationships between transcription factor binding sites. While ATF-2, c-Jun, and the coactivator proteins CBP/p300 play a central role in TNF-alpha gene activation stimulated by virus infection or intracellular calcium flux, different sets of activators including NFATp, Sp1, and Ets/Elk are recruited to a shared set of transcription factor binding sites depending upon the particular stimulus. Thus, these studies demonstrate that the inducer-specific assembly of unique enhanceosomes is a general mechanism by which a single gene is controlled in response to different extracellular stimuli.
Figures








References
-
- Arias, J., A. S. Alberts, P. Brindle, F. X. Claret, T. Smeal, M. Karin, J. Feramisco, and M. Montminy. 1994. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature 370:226-229. - PubMed
-
- Bassuk, A. G., and J. M. Leiden. 1995. A direct physical association between ETS and AP-1 transcription factors in normal human T cells. Immunity 3:223-237. - PubMed
-
- Billon, N., D. Carlisi, M. B. Datto, L. A. van Grunsven, A. Watt, X.-F. Wang, and B. B. Rudkin. 1999. Cooperation of Sp1 and p300 in the induction of the CDK inhibitor p21WAF1/CIP1 during NGF-mediated neuronal differentiation. Oncogene 18:2872-2882. - PubMed
-
- Block, K. L., Y. Shou, and M. Poncz. 1996. An Ets/Sp1 interaction in the 5′-flanking region of the megakaryocyte-specific alpha IIb gene appears to stabilize Sp1 binding and is essential for expression of this TATA-less gene. Blood 88:2071-2080. - PubMed
-
- Brinkman, B. M., J. B. Telliez, A. R. Schievella, L. L. Lin, and A. E. Goldfeld. 1999. Engagement of tumor necrosis factor (TNF) receptor 1 leads to ATF-2- and p38 mitogen-activated protein kinase-dependent TNF-alpha gene expression. J. Biol. Chem. 274:30882-30886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
