Stat1-vitamin D receptor interactions antagonize 1,25-dihydroxyvitamin D transcriptional activity and enhance stat1-mediated transcription
- PMID: 11909970
- PMCID: PMC133712
- DOI: 10.1128/MCB.22.8.2777-2787.2002
Stat1-vitamin D receptor interactions antagonize 1,25-dihydroxyvitamin D transcriptional activity and enhance stat1-mediated transcription
Abstract
The cytokine gamma interferon (IFN-gamma) and the calcitropic steroid hormone 1,25-dihydroxyvitamin D (1,25D) are activators of macrophage immune function. In sarcoidosis, tuberculosis, and several granulomatoses, IFN-gamma induces 1,25D synthesis by macrophages and inhibits 1,25D induction of 24-hydroxylase, a key enzyme in 1,25D inactivation, causing high levels of 1,25D in serum and hypercalcemia. This study delineates IFN-gamma-1,25D cross talk in human monocytes-macrophages. Nuclear accumulation of Stat1 and vitamin D receptor (VDR) by IFN-gamma and 1,25D promotes protein-protein interactions between Stat1 and the DNA binding domain of the VDR. This prevents VDR-retinoid X receptor (RXR) binding to the vitamin D-responsive element, thus diverting the VDR from its normal genomic target on the 24-hydroxylase promoter and antagonizing 1,25D-VDR transactivation of this gene. In contrast, 1,25D enhances IFN-gamma action. Stat1-VDR interactions, by preventing Stat1 deactivation by tyrosine dephosphorylation, cooperate with IFN-gamma/Stat1-induced transcription. This novel 1,25D-IFN-gamma cross talk explains the pathogenesis of abnormal 1,25D homeostasis in granulomatous processes and provides new insights into 1,25D immunomodulatory properties.
Figures

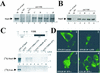
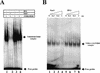
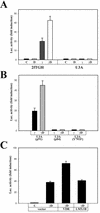



References
-
- Adams, J. S., M. A. Gacad, A. Anders, D. B. Endres, and O. P. Sharma. 1986. Biochemical indicators of disordered vitamin D and calcium homeostasis in sarcoidosis. Sarcoidosis 3:1-6. - PubMed
-
- Adams, J. S., R. L. Modlin, M. M. Diz, and P. F. Barnes. 1989. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J. Clin. Endocrinol. Metab. 69:457-460. - PubMed
-
- Adams, J. S., F. R. Singer, M. A. Gacad, O. P. Sharma, M. J. Hayes, P. Vouros, and M. F. Holick. 1985. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J. Clin. Endocrinol. Metab. 60:960-966. - PubMed
-
- Aittomaki, S., M. Pesu, B. Groner, O. A. Janne, J. J. Palvimo, and O. Silvennoinen. 2000. Cooperation among Stat1, glucocorticoid receptor, and PU.1 in transcriptional activation of the high-affinity Fc gamma receptor I in monocytes. J. Immunol. 164:5689-5697. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
