Requirement of TRAP/mediator for both activator-independent and activator-dependent transcription in conjunction with TFIID-associated TAF(II)s
- PMID: 11909976
- PMCID: PMC133729
- DOI: 10.1128/MCB.22.8.2842-2852.2002
Requirement of TRAP/mediator for both activator-independent and activator-dependent transcription in conjunction with TFIID-associated TAF(II)s
Abstract
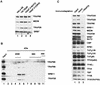
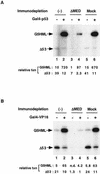
The multiprotein human TRAP/Mediator complex, which is phylogenetically related to the yeast SRB/Mediator coactivator, facilitates activation through a wide variety of transcriptional activators. However, it remains unclear how TRAP/Mediator functions in the context of other coactivators. Here we have identified a previously uncharacterized integral subunit (TRAP25) of the complex that is apparently metazoan specific. An antibody that is specific for TRAP25 allowed quantitative immunodepletion of essentially all TRAP/Mediator components from HeLa nuclear extract, without detectably affecting levels of RNA polymerase II and corresponding general transcription factors. Surprisingly, the TRAP/Mediator-depleted nuclear extract displayed severely reduced levels of both basal and activator-dependent transcription from DNA templates. Both activities were efficiently restored upon readdition of purified TRAP/Mediator. Moreover, restoration of basal and activator-dependent transcription to extracts that were simultaneously depleted of TRAP/Mediator and TFIID (TBP plus the major TAF(II)s) required addition of both TBP and associated TAF(II)s, as well as TRAP/Mediator. These observations indicate that TAF(II)s and Mediator are jointly required for both basal and activated transcription in the context of a more physiological complement of nuclear proteins. We propose a close mechanistic linkage between these components that most likely operates at the level of combined effects on the general transcription machinery and, in addition, a direct role for Mediator in relaying activation signals to this machinery.
Figures





References
-
- Akoulitchev, S., S. Chuikov, and D. Reinberg. 2000. TFIIH is negatively regulated by cdk8-containing mediator complexes. Nature 407:102-106. - PubMed
-
- Boyer, T. G., M. E. D. Martin, E. Lees, R. P. Ricciard, and A. P. Berk. 1999. Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature 399:276-279. - PubMed
-
- Burke, T. W., and J. T. Kadonaga. 1996. Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 10:711-724. - PubMed
-
- Burley, S. K., and R. G. Roeder. 1996. Biochemistry and structural biology of transcription factor IID (TFIID). Annu. Rev. Biochem. 65:769-799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
