NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer
- PMID: 11909978
- PMCID: PMC133743
- DOI: 10.1128/MCB.22.8.2862-2870.2002
NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer
Abstract
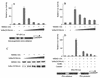
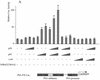
The transcription factor NF-kappa B regulates gene expression involved in cell growth and survival and has been implicated in progression of hormone-independent breast cancer. By expressing a dominant-active form of mitogen-activated protein kinase kinase kinase 1, by exposure to tumor necrosis factor alpha, or by overexpression of p50/p65, we show that NF-kappa B activates a transcription regulatory element of the prostate-specific antigen (PSA)-encoding gene, a marker for prostate cancer development, treatment, and progression. By DNase I footprinting, we identified four NF-kappa B binding sites in the PSA core enhancer. We also demonstrate that androgen-independent prostate cancer xenografts have higher constitutive NF-kappa B binding activity than their androgen-dependent counterparts. These results suggest a role of NF-kappa B in prostate cancer progression.
Figures
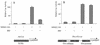









References
-
- Aarnisalo, P., H. Santti, H. Poukka, J. J. Palvimo, and O. A. Janne. 1999. Transcription activating and repressing functions of the androgen receptor are differentially influenced by mutations in the deoxyribonucleic acid binding domain. Endocrinology 140:3097-3105. - PubMed
-
- Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. - PubMed
-
- Chadwick, C. C., L. J. Shaw, and R. C. Winneker. 1998. TNF-α and 9-cis-retinoic acid synergistically induce ICAM-1 expression: evidence for interaction of retinoid receptors with NF-κB. Exp. Cell Res. 239:423-429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
