Rice SPK, a calmodulin-like domain protein kinase, is required for storage product accumulation during seed development: phosphorylation of sucrose synthase is a possible factor
- PMID: 11910009
- PMCID: PMC150584
- DOI: 10.1105/tpc.010454
Rice SPK, a calmodulin-like domain protein kinase, is required for storage product accumulation during seed development: phosphorylation of sucrose synthase is a possible factor
Abstract
Suc, an end product of photosynthesis, is metabolized by Suc synthase in sink organs as an initial step in the biosynthesis of storage products. Suc synthase activity is known to be regulated by reversible phosphorylation, but the details of this process are unclear at present. Rice SPK, a calcium-dependent protein kinase, is expressed uniquely in the endosperm of immature seed, and its involvement in the biosynthetic pathways of storage products was suggested. Antisense SPK transformants lacked the ability to accumulate storage products such as starch, but produced watery seed with a large amount of Suc instead, as the result of an inhibition of Suc degradation. Analysis of in vitro phosphorylation indicated that SPK phosphorylated specifically a Ser residue in Suc synthase that has been shown to be important for its activity in the degradation of Suc. This finding suggests that SPK is involved in the activation of Suc synthase. It appears that SPK is a Suc synthase kinase that may be important for supplying substrates for the biosynthesis of storage products.
Figures
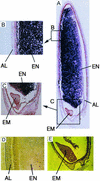

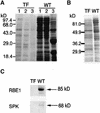
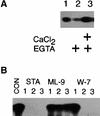
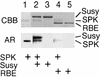


References
-
- Ausubel, E.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K., eds (1987). Current Protocols in Molecular Biology. (New York: John Wiley & Sons).
-
- Camoni, L., Harper, J.F., and Palmgren, M.G. (1998). 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK). FEBS Lett. 430, 381–384. - PubMed
-
- Chikano, H., Ogawa, M., Ikeda, Y., Koizumi, N., Kusano, T., and Sano, H. (2001). Two novel genes encoding SNF1-related protein kinases from Arabidopsis thaliana: Differential accumulation of AtSR1 and AtSR2 transcripts in response to cytokinins and sugars, and phosphorylation of sucrose synthase by AtSR2. Mol. Gen. Genet. 264, 674–681. - PubMed
-
- Chourey, R.S., Talircio, W.W., Carlson, S.J., and Ruan, Y.-L. (1998). Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol. Gen. Genet. 259, 88–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

