Complex behavior in solution of homodimeric SecA
- PMID: 11910030
- PMCID: PMC2373524
- DOI: 10.1110/ps.4090102
Complex behavior in solution of homodimeric SecA
Abstract
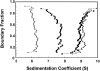
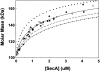
SecA, a homodimeric protein involved in protein export in Escherichia coli, exists in the cell both associated with the membrane translocation apparatus and free in the cytosol. SecA is a multifunctional protein involved in protein localization and regulation of its own expression. To carry out these functions, SecA interacts with a variety of proteins, phospholipids, nucleotides, and nucleic acid and shows two enzymic activities. It is an ATPase and a helicase. Its role during protein localization involves interaction with the precursor polypeptides to be exported, the cytosolic chaperone SecB, and the SecY subunit of the membrane-associated translocase, as well as with acidic phospholipids. At the membrane, SecA undergoes a cycle of binding and hydrolysis of ATP coupled to conformational changes that result in translocation of precursors through the cytoplasmic membrane. The helicase activity of SecA and its affinity for its mRNA are involved in regulation of its own expression. SecA has been reported to exist in at least two conformational states during its functional cycle. Here we have used analytical centrifugation, as well as column chromatography coupled with multi-angle light scatter, to show that in solution SecA undergoes at least two monomer-dimer equilibrium reactions that are sensitive to temperature and to concentration of salt.
Figures





Similar articles
-
Sec-dependent preprotein translocation in bacteria.Arch Microbiol. 1996 Jan;165(1):1-8. doi: 10.1007/s002030050289. Arch Microbiol. 1996. PMID: 8639021 Review.
-
Sites of interaction between SecA and the chaperone SecB, two proteins involved in export.Protein Sci. 2004 Apr;13(4):1124-33. doi: 10.1110/ps.03410104. Epub 2004 Mar 9. Protein Sci. 2004. PMID: 15010547 Free PMC article.
-
Binding, activation and dissociation of the dimeric SecA ATPase at the dimeric SecYEG translocase.EMBO J. 2003 Sep 1;22(17):4375-84. doi: 10.1093/emboj/cdg418. EMBO J. 2003. PMID: 12941690 Free PMC article.
-
The ATPase domain of SecA can form a tetramer in solution.J Mol Biol. 2002 Jan 25;315(4):831-43. doi: 10.1006/jmbi.2001.5279. J Mol Biol. 2002. PMID: 11812151
-
SecA: the ubiquitous component of preprotein translocase in prokaryotes.Microbes Infect. 1999 Oct;1(12):993-1004. doi: 10.1016/s1286-4579(99)80517-6. Microbes Infect. 1999. PMID: 10617931 Review.
Cited by
-
The oligomeric state and arrangement of the active bacterial translocon.J Biol Chem. 2011 Feb 11;286(6):4659-69. doi: 10.1074/jbc.M110.175638. Epub 2010 Nov 5. J Biol Chem. 2011. PMID: 21056980 Free PMC article.
-
The use of analytical sedimentation velocity to extract thermodynamic linkage.Biophys Chem. 2011 Nov;159(1):120-8. doi: 10.1016/j.bpc.2011.05.014. Epub 2011 May 27. Biophys Chem. 2011. PMID: 21703752 Free PMC article.
-
SecA functions in vivo as a discrete anti-parallel dimer to promote protein transport.Mol Microbiol. 2017 Feb;103(3):439-451. doi: 10.1111/mmi.13567. Epub 2016 Dec 7. Mol Microbiol. 2017. PMID: 27802584 Free PMC article.
-
Direct visualization of the E. coli Sec translocase engaging precursor proteins in lipid bilayers.Sci Adv. 2019 Jun 12;5(6):eaav9404. doi: 10.1126/sciadv.aav9404. eCollection 2019 Jun. Sci Adv. 2019. PMID: 31206019 Free PMC article.
-
Probing the affinity of SecA for signal peptide in different environments.Biochemistry. 2005 Oct 25;44(42):13987-96. doi: 10.1021/bi050882k. Biochemistry. 2005. PMID: 16229488 Free PMC article.
References
-
- Akita, M., Shinkai, A., Matsuyama, S., and Mizushima, S. 1991. SecA, an essential component of the secretory machinery of Escherichia coli, exists as homodimer. Biochem. Biophys. Res. Commun. 174 211–216. - PubMed
-
- Cabelli, R.J., Dolan, K.M., Qian, L.P., and Oliver, D.B. 1991. Characterization of membrane-associated and soluble states of SecA protein from wild-type and SecA51(TS) mutant strains of Escherichia coli. J. Biol. Chem. 266 24420–24427. - PubMed
-
- Castle, A.M., Macnab, R.M., and Shulman, R.G. 1986. Coupling between the sodium and proton gradients in respiring Escherichia coli cells measured by 23Na and 31P nuclear magnetic resonance. J. Biol. Chem. 261 7797–7806. - PubMed
-
- Cayley, S., Lewis, B.A., Guttman, H.J., and Record, M.T., Jr. 1991. Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. Implications for protein-DNA interactions in vivo. J. Mol. Biol. 222 281–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

