Subunit composition of a bicomponent toxin: staphylococcal leukocidin forms an octameric transmembrane pore
- PMID: 11910032
- PMCID: PMC2373538
- DOI: 10.1110/ps.4360102
Subunit composition of a bicomponent toxin: staphylococcal leukocidin forms an octameric transmembrane pore
Abstract
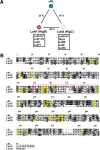
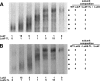
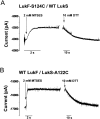
Staphylococcal leukocidin pores are formed by the obligatory interaction of two distinct polypeptides, one of class F and one of class S, making them unique in the family of beta-barrel pore-forming toxins (beta-PFTs). By contrast, other beta-PFTs form homo-oligomeric pores; for example, the staphylococcal alpha-hemolysin (alpha HL) pore is a homoheptamer. Here, we deduce the subunit composition of a leukocidin pore by two independent methods: gel shift electrophoresis and site-specific chemical modification during single-channel recording. Four LukF and four LukS subunits coassemble to form an octamer. This result in part explains properties of the leukocidin pore, such as its high conductance compared to the alpha HL pore. It is also pertinent to the mechanism of assembly of beta-PFT pores and suggests new possibilities for engineering these proteins.
Figures




References
-
- Abrahams, J.P., Leslie, A.G.W., Lutter, R., and Walker, J.E. 1994. Structure at 2.8Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370 621–628. - PubMed
-
- Alouf, J.E. and J.H. Freer. 1999. The comprehensive sourcebook of bacterial protein toxins, 2nd ed. Academic Press, San Diego, CA, p. 718.
-
- Archibald, J.M., Logsdon, J.M., and Doolittle, W.F. 2000. Origin and evolution of eukaryotic chaperonins: Phylogenetic evidence for ancient duplications in CCT genes. Mol. Biol. Evol. 17 1456–1466. - PubMed
-
- Baida, G., Budarina, Z.I., Kuzmin, N.P., and Solonin, A.S. 1999. Complete nucleotide sequence and molecular characterization of hemolysin II gene from Bacillus cereus. FEMS Microbiol. Lett. 180 7–14. - PubMed
-
- Bayley, H. 1997. Toxin structure: Part of a hole? Current Biol. 7 R763–R767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

