Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties
- PMID: 11914275
- PMCID: PMC155359
- DOI: 10.1101/gad.952602
Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties
Abstract
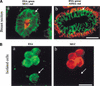
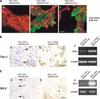
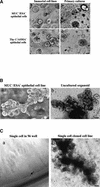
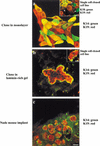
The epithelial compartment of the human breast comprises two distinct lineages: the luminal epithelial and the myoepithelial lineage. We have shown previously that a subset of the luminal epithelial cells could convert to myoepithelial cells in culture signifying the possible existence of a progenitor cell. We therefore set out to identify and isolate the putative precursor in the luminal epithelial compartment. Using cell surface markers and immunomagnetic sorting, we isolated two luminal epithelial cell populations from primary cultures of reduction mammoplasties. The major population coexpresses sialomucin (MUC(+)) and epithelial-specific antigen (ESA(+)) whereas the minor population has a suprabasal position and expresses epithelial specific antigen but no sialomucin (MUC(-)/ESA(+)). Two cell lines were further established by transduction of the E6/E7 genes from human papilloma virus type 16. Both cell lines maintained a luminal epithelial phenotype as evidenced by expression of the tight junction proteins, claudin-1 and occludin, and by generation of a high transepithelial electrical resistance on semipermeable filters. Whereas in clonal cultures, the MUC(+)/ESA(+) epithelial cell line was luminal epithelial restricted in its differentiation repertoire, the suprabasal-derived MUC(-)/ESA(+) epithelial cell line was able to generate itself as well as MUC(+)/ESA(+) epithelial cells and Thy-1(+)/alpha-smooth muscle actin(+) (ASMA(+)) myoepithelial cells. The MUC(-)/ESA(+) epithelial cell line further differed from the MUC(+)/ESA(+) epithelial cell line by the expression of keratin K19, a feature of a subpopulation of epithelial cells in terminal duct lobular units in vivo. Within a reconstituted basement membrane, the MUC(+)/ESA(+) epithelial cell line formed acinus-like spheres. In contrast, the MUC(-)/ESA(+) epithelial cell line formed elaborate branching structures resembling uncultured terminal duct lobular units both by morphology and marker expression. Similar structures were obtained by inoculating the extracellular matrix-embedded cells subcutaneously in nude mice. Thus, MUC(-)/ESA(+) epithelial cells within the luminal epithelial lineage may function as precursor cells of terminal duct lobular units in the human breast.
Figures







References
-
- Anbazhagan R, Osin PP, Bartkova J, Nathan B, Lane EB, Gusterson BA. The development of epithelial phenotypes in the human fetal and infant breast. J Pathol. 1998;184:197–206. - PubMed
-
- Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J Mol Med. 1999;77:699–712. - PubMed
-
- Band V. Preneoplastic transformation of human mammary epithelial cells. Semin Cancer Biol. 1995;6:185–192. - PubMed
-
- ————— The role of retinoblastoma and p53 tumor suppressor pathways in human mammary epithelial cell immortalization. Int J Oncol. 1998;12:499–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
