Enterococcus faecalis acetoacetyl-coenzyme A thiolase/3-hydroxy-3-methylglutaryl-coenzyme A reductase, a dual-function protein of isopentenyl diphosphate biosynthesis
- PMID: 11914342
- PMCID: PMC134966
- DOI: 10.1128/JB.184.8.2116-2122.2002
Enterococcus faecalis acetoacetyl-coenzyme A thiolase/3-hydroxy-3-methylglutaryl-coenzyme A reductase, a dual-function protein of isopentenyl diphosphate biosynthesis
Abstract
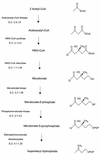
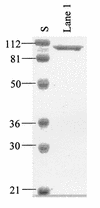
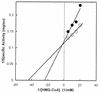
Many bacteria employ the nonmevalonate pathway for synthesis of isopentenyl diphosphate, the monomer unit for isoprenoid biosynthesis. However, gram-positive cocci exclusively use the mevalonate pathway, which is essential for their growth (E. I. Wilding et al., J. Bacteriol. 182:4319-4327, 2000). Enzymes of the mevalonate pathway are thus potential targets for drug intervention. Uniquely, the enterococci possess a single open reading frame, mvaE, that appears to encode two enzymes of the mevalonate pathway, acetoacetyl-coenzyme A thiolase and 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Western blotting revealed that the mvaE gene product is a single polypeptide in Enterococcus faecalis, Enterococcus faecium, and Enterococcus hirae. The mvaE gene was cloned from E. faecalis and was expressed with an N-terminal His tag in Escherichia coli. The gene product was then purified by nickel affinity chromatography. As predicted, the 86.5-kDa mvaE gene product catalyzed both the acetoacetyl-CoA thiolase and HMG-CoA reductase reactions. Temperature optima, DeltaH(a) and K(m) values, and pH optima were determined for both activities. Kinetic studies of acetoacetyl-CoA thiolase implicated a ping-pong mechanism. CoA acted as an inhibitor competitive with acetyl-CoA. A millimolar K(i) for a statin drug confirmed that E. faecalis HMG-CoA reductase is a class II enzyme. The oxidoreductant was NADP(H). A role for an active-site histidine during the first redox step of the HMG-CoA, reductase reaction was suggested by the ability of diethylpyrocarbonate to block formation of mevalonate from HMG-CoA, but not from mevaldehyde. Sequence comparisons with other HMG-CoA reductases suggest that the essential active-site histidine is His756. The mvaE gene product represents the first example of an HMG-CoA reductase fused to another enzyme.
Figures
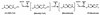


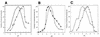


References
-
- Bochar, D. A., C. V. Stauffacher, and V. W. Rodwell. 1999. Sequence comparisons reveal two classes of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol. Genet. Metab. 6:122-127. - PubMed
-
- Bochar, D. A., J. A. Friesen, C. V. Stauffacher, and V. W. Rodwell. 1999. Biosynthesis of mevalonic acid from acetyl-CoA, p. 15-44. In David Cane (ed.), Comprehensive natural products chemistry, vol. 2. Pergamon Press, Oxford, United Kingdom.
-
- Clinkenbeard, K. D., T. Sugiyama, and M. D. Lane. 1975. Cystolic acetoacetyl-CoA thiolase from chicken liver. Methods Enzymol. 35:167-173. - PubMed
-
- Corsini, A., F. M. Maggi, and A. L. Catapano. 1995. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol. Res. 31:9-27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

