The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus
- PMID: 11916669
- PMCID: PMC123826
- DOI: 10.1128/AEM.68.4.1561-1568.2002
The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus
Abstract
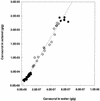
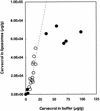
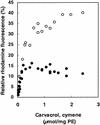
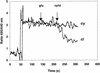
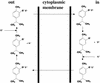
The natural antimicrobial compound carvacrol shows a high preference for hydrophobic phases. The partition coefficients of carvacrol in both octanol-water and liposome-buffer phases were determined (3.64 and 3.26, respectively). Addition of carvacrol to a liposomal suspension resulted in an expansion of the liposomal membrane. Maximum expansion was observed after the addition of 0.50 micromol of carvacrol/mg of L-alpha-phosphatidylethanolamine. Cymene, a biological precursor of carvacrol which lacks a hydroxyl group, was found to have a higher preference for liposomal membranes, thereby causing more expansion. The effect of cymene on the membrane potential was less pronounced than the effect of carvacrol. The pH gradient and ATP pools were not affected by cymene. Measurement of the antimicrobial activities of compounds similar to carvacrol (e.g., thymol, cymene, menthol, and carvacrol methyl ester) showed that the hydroxyl group of this compound and the presence of a system of delocalized electrons are important for the antimicrobial activity of carvacrol. Based on this study, we hypothesize that carvacrol destabilizes the cytoplasmic membrane and, in addition, acts as a proton exchanger, thereby reducing the pH gradient across the cytoplasmic membrane. The resulting collapse of the proton motive force and depletion of the ATP pool eventually lead to cell death.
Figures

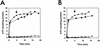

References
-
- Arrebola, M. L., M. C. Navarro, J. Jiménez, and F. A. Ocaña. 1994. Yield and composition of the essential oil of Thymus serpylloides subsp. serpylloides. Phytochemistry 36:67-72.
-
- Banerjee, S., S. H. Yalkowsky, and S. C. Valvani. 1980. Water solubility and octanol/water partition coefficients of organics. Limitations of the solubility-partition coefficient correlation. Environ. Sci. Technol. 14:1227-1229.
-
- Bose, S. M., C. N. BhimaRao, and V. Subramanyan. 1949. Influence of organic matter on bactericidal efficiency of Indian essential oils. J. Sci. Ind. Res. 8:157.
-
- Brown, W. H. 1975. Introduction to organic chemistry, 3rd ed. Willard Grant Press, Boston, Mass.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

