Use of multiplex PCR and PCR restriction enzyme analysis for detection and exploration of the variability in the free-living amoeba Naegleria in the environment
- PMID: 11916734
- PMCID: PMC123847
- DOI: 10.1128/AEM.68.4.2061-2065.2002
Use of multiplex PCR and PCR restriction enzyme analysis for detection and exploration of the variability in the free-living amoeba Naegleria in the environment
Abstract
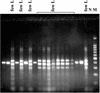
A multiplex PCR was developed to simultaneously detect Naegleria fowleri and other Naegleria species in the environment. Multiplex PCR was also capable of identifying N. fowleri isolates with internal transcribed spacers of different sizes. In addition, restriction fragment length polymorphism analysis of the PCR product distinguished the main thermophilic Naegleria species from the sampling sites.
Figures


References
-
- Adams, M., R. H. Andrews, B. Robinson, P. Christy, P. R. Baverstok, P. J. Dobson, and S. J. Blackler. 1989. A genetic approach to species criteria in the amoeba genus Naegleria using allozyme electrophoresis. Int. J. Parasitol. 19:823-834. - PubMed
-
- Brown, M. R., and J. Barker. 1999. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol. 7:46-50. - PubMed
-
- Carter, R. F. 1970. Description of a Naegleria sp. isolated from two cases of primary amoebic meningo-encephalitis, and of the experimental pathological changes induced by it. J. Pathol. 100:217-244. - PubMed
-
- Cerva, L., W. Kasprzak, and T. Mazur. 1982. Naegleria fowleri in cooling waters of power plants. J. Hyg. Epidemiol. Microbiol. Immunol. 26:152-161. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

