Endothelial cells derived from human embryonic stem cells
- PMID: 11917100
- PMCID: PMC123658
- DOI: 10.1073/pnas.032074999
Endothelial cells derived from human embryonic stem cells
Abstract
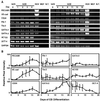
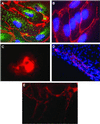
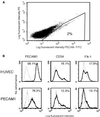
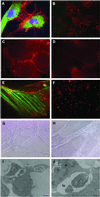
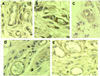
Human embryonic stem cells have the potential to differentiate into various cell types and, thus, may be useful as a source of cells for transplantation or tissue engineering. We describe here the differentiation steps of human embryonic stem cells into endothelial cells forming vascular-like structures. The human embryonic-derived endothelial cells were isolated by using platelet endothelial cell-adhesion molecule-1 (PECAM1) antibodies, their behavior was characterized in vitro and in vivo, and their potential in tissue engineering was examined. We show that the isolated embryonic PECAM1+ cells, grown in culture, display characteristics similar to vessel endothelium. The cells express endothelial cell markers in a pattern similar to human umbilical vein endothelial cells, their junctions are correctly organized, and they have high metabolism of acetylated low-density lipoprotein. In addition, the cells are able to differentiate and form tube-like structures when cultured on matrigel. In vivo, when transplanted into SCID mice, the cells appeared to form microvessels containing mouse blood cells. With further studies, these cells could provide a source of human endothelial cells that could be beneficial for potential applications such as engineering new blood vessels, endothelial cell transplantation into the heart for myocardial regeneration, and induction of angiogenesis for treatment of regional ischemia.
Figures

References
-
- Niklason L E, Gao J, Abbott W M, Hirschi K K, Houser S, Marini R, Langer R. Science. 1999;284:489–493. - PubMed
-
- Kawamoto A, Gwon H C, Iwaguro H, Yamaguchi J I, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner J M, Asahara T. Circulation. 2001;103:634–637. - PubMed
-
- Vittet D, Prandini M H, Berthier R, Schweitzer A, Martin-Sisteron H, Uzan G, Dejana E. Blood. 1996;88:3424–3431. - PubMed
-
- Yancopoulos G D, Klagsbrun M, Folkman J. Cell. 1998;93:661–664. - PubMed
-
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K. Nature (London) 2000;408:92–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

