A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP
- PMID: 11917117
- PMCID: PMC123710
- DOI: 10.1073/pnas.072033799
A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP
Abstract
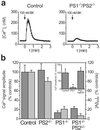
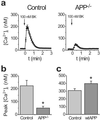
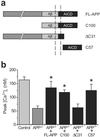
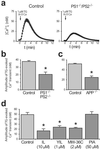
Presenilins mediate an unusual intramembranous proteolytic activity known as gamma-secretase, two substrates of which are the Notch receptor (Notch) and the beta-amyloid precursor protein (APP). Gamma-secretase-mediated cleavage of APP, like that of Notch, yields an intracellular fragment [APP intracellular domain (AICD)] that forms a transcriptively active complex. We now demonstrate a functional role for AICD in regulating phosphoinositide-mediated calcium signaling. Genetic ablation of the presenilins or pharmacological inhibition of gamma-secretase activity (and thereby AICD production) attenuated calcium signaling in a dose-dependent and reversible manner through a mechanism involving the modulation of endoplasmic reticulum calcium stores. Cells lacking APP (and hence AICD) exhibited similar calcium signaling deficits, and-notably-these disturbances could be reversed by transfection with APP constructs containing an intact AICD, but not by constructs lacking this domain. Our findings indicate that the AICD regulates phosphoinositide-mediated calcium signaling through a gamma-secretase-dependent signaling pathway, suggesting that the intramembranous proteolysis of APP may play a signaling role analogous to that of Notch.
Figures

References
-
- Wolfe M S, Haass C. J Biol Chem. 2001;276:5413–5416. - PubMed
-
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm J S, Schroeter E H, Schrijvers V, Wolfe M S, Ray W J, et al. Nature (London) 1999;398:518–522. - PubMed
-
- Artavanis-Tsakonas S, Rand M D, Lake R J. Science. 1999;284:770–776. - PubMed
-
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens J R, Cumano A, Roux P, Black R A, Israël A. Mol Cell. 2000;5:207–216. - PubMed
-
- Greenwald I. Genes Dev. 1998;12:1751–1762. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

