Desensitization of mu-opioid receptor-evoked potassium currents: initiation at the receptor, expression at the effector
- PMID: 11917119
- PMCID: PMC123706
- DOI: 10.1073/pnas.072075399
Desensitization of mu-opioid receptor-evoked potassium currents: initiation at the receptor, expression at the effector
Abstract
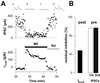
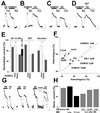
Many G protein-coupled receptor-mediated responses desensitize within minutes. Sustained stimulation of mu-opioid receptors (MORs), which primarily signal through G(i/o) proteins, leads to activation and subsequent desensitization of G protein-coupled inwardly rectifying potassium (GIRK) currents. We observed that in neurons of the locus coeruleus, which express among the highest levels of MORs in the brain, the degree of desensitization depended on the intensity of receptor stimulation, indicating that the process is initiated at the receptor. Interestingly, while GIRK-mediated postsynaptic inhibition substantially desensitized within 15 min, presynaptic inhibition of afferent transmission, which involves other effector systems, remained constant, suggesting that the postsynaptic desensitization we observed is expressed at the effector. We show that desensitized GIRK currents can gradually be reactivated by additional G protein signals of increasing intensity and present evidence that desensitization is a G protein-mediated process. Finally, desensitization of MOR-induced GIRK currents had heterologous effects on responses mediated by other G protein-coupled receptors converging onto the same population of GIRK channels. Taken together, our results provide evidence for a form of desensitization mediated by a slowly developing G protein-dependent pathway, initiated at the MORs and leading to competitive inhibition of GIRK channel activation. This implies that MORs exert a bidirectional action on GIRK channels.
Figures



References
-
- Matthes H W, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, et al. Nature (London) 1996;383:819–823. - PubMed
-
- Reuveny E, Slesinger P A, Inglese J, Morales J M, Iniguez-Lluhi J A, Lefkowitz R J, Bourne H R, Jan Y N, Jan L Y. Nature (London) 1994;370:143–146. - PubMed
-
- Huang C L, Slesinger P A, Casey P J, Jan Y N, Jan L Y. Neuron. 1995;15:1133–1143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

