Helicogenicity of solvents in the conformational equilibrium of oligo(m-phenylene ethynylene)s: implications for foldamer research
- PMID: 11917139
- PMCID: PMC122720
- DOI: 10.1073/pnas.072642799
Helicogenicity of solvents in the conformational equilibrium of oligo(m-phenylene ethynylene)s: implications for foldamer research
Abstract
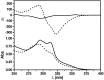
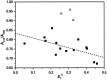
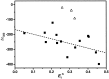
A (R)-binaphthol tethered bis-hexameric oligo(m-phenylene ethynylene) foldamer was examined in 30 solvents to correlate the unfolded-folded conformational equilibrium to bulk solvent properties and specific solvent-chain interactions. The oligomer is soluble in a variety of solvents of intermediate polarity, with the majority of these solvents being helicogenic. The amphiphilic nature of the chain allows the solvophobic backbone to be solubilized in a wide range of solvents through the polar triethylene glycol side chains. As demonstrated through UV and CD spectroscopic experiments, the helical conformation is increasingly stabilized with increasing solvent polarity in the absence of specific solvent-chain interactions. Surprisingly, very few solvents are capable of fully denaturing the helix, indicating the strength of the solvophobic driving forces in this cooperative system. The folding reaction for this amphiphilic oligomer can be described as a compromise in solubility properties, where chains collapse intramolecularly into helical conformations to minimize solvent-backbone contacts while maintaining favorable solvent-side chain interactions for solvation. In terms of mimicking the properties of biomacromolecules, foldamers using solvophobic driving forces must be tempered with functionalities that promote solubility of the folded state while at the same time allowing access to the unfolded state through the use of denaturants.
Figures

References
-
- Kauzman W. Adv Protein Chem. 1959;14:1–63. - PubMed
-
- Williams C, Brochard F, Frisch H L. Annu Rev Phys Chem. 1981;32:433–451.
-
- Dill K A. Biochemistry. 1990;29:7133–7155. - PubMed
-
- Chan H S, Dill K A. Macromolecules. 1989;22:4559–4573.
-
- Camacho C J, Thirumalai D. Phys Rev Lett. 1993;71:2505–2508. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

