Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices
- PMID: 11923408
- PMCID: PMC6758296
- DOI: 10.1523/JNEUROSCI.22-07-02443.2002
Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices
Abstract
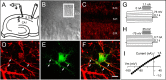
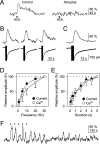
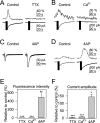
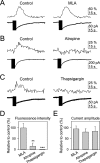
Recent results have demonstrated the existence of bidirectional communication between glial cells and neurons. We investigated in brain slices whether rat hippocampal astrocytes respond to acetylcholine synaptically released by an extrinsic pathway. We stimulated the stratum oriens/alveus, which contains cholinergic afferents from the septum and diagonal band of Broca, and recorded whole-cell membrane currents and intracellular Ca2+ levels of astrocytes located in the hippocampal stratum oriens. Nerve-fiber stimulation evoked a long-lasting inward current and increased the Ca2+ levels in astrocytes. Both astrocytic responses were abolished by tetrodotoxin or Cd2+ and were increased by 4-aminopyridine, indicating that the responses were attributable to synaptically released neurotransmitter. The inward current was inhibited by glutamate transporter antagonists, indicating that it was attributable to the electrogenic glutamate transporter activity. The synaptically evoked intracellular Ca2+ elevations were not affected by glutamate receptor antagonists but were abolished by atropine, indicating that they were mediated by muscarinic cholinergic receptors. Thapsigargin prevented the Ca2+ elevation but did not modify the inward current, indicating that the Ca2+ signal was attributable to intracellular Ca2+ mobilization. These results indicate that hippocampal astrocytes respond to acetylcholine released by synaptic terminals. The synaptically released acetylcholine acts on muscarinic receptors, mobilizing Ca2+ from the intracellular stores. Different regions in the recorded astrocytes showed independent stimulus-induced Ca2+ variations, suggesting the existence of subcellular domains in the astrocytic responses evoked by the synaptic cholinergic activity. Therefore, our results show the existence of cholinergic neuron-astrocyte signaling and suggest that astrocytes are a target of axonal inputs from different brain areas.
Figures


References
-
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The rat nervous system, Ed 2. Academic; London: 1995. pp. 443–493.
-
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998a;10:2129–2142. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
