Disease-specific human glycine receptor alpha1 subunit causes hyperekplexia phenotype and impaired glycine- and GABA(A)-receptor transmission in transgenic mice
- PMID: 11923415
- PMCID: PMC6758287
- DOI: 10.1523/JNEUROSCI.22-07-02505.2002
Disease-specific human glycine receptor alpha1 subunit causes hyperekplexia phenotype and impaired glycine- and GABA(A)-receptor transmission in transgenic mice
Abstract
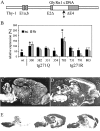
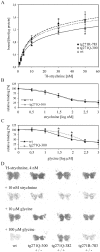
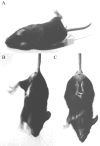
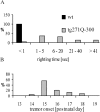
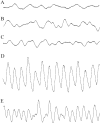
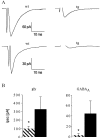
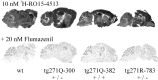
Hereditary hyperekplexia is caused by disinhibition of motoneurons resulting from mutations in the ionotropic receptor for the inhibitory neurotransmitter glycine (GlyR). To study the pathomechanisms involved in vivo, we generated and analyzed transgenic mice expressing the hyperekplexia-specific dominant mutant human GlyR alpha1 subunit 271Q. Tg271Q transgenic mice, in contrast to transgenic animals expressing a wild-type human alpha1 subunit (tg271R), display a dramatic phenotype similar to spontaneous and engineered mouse mutations expressing reduced levels of GlyR. Electrophysiological analysis in the ventral horn of the spinal cord of tg271Q mice revealed a diminished GlyR transmission. Intriguingly, an even larger reduction was found for GABA(A)-receptor-mediated inhibitory transmission, indicating that the expression of this disease gene not only affects the glycinergic system but also leads to a drastic downregulation of the entire postsynaptic inhibition. Therefore, the transgenic mice generated here provide a new animal model of systemic receptor interaction to study inherited and acquired neuromotor deficiencies at different functional levels and to develop novel therapeutic concepts for these diseases.
Figures
References
-
- Andrew M, Owen MJ. Hyperekplexia: abnormal startle response due to glycine receptor mutations. Br J Psychiatry. 1997;170:106–108. - PubMed
-
- Becker CM, Schmieden V, Tarroni P, Strasser U, Betz H. Isoform-selective deficit of glycine receptors in the mouse mutant spastic. Neuron. 1992;8:283–289. - PubMed
-
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
