Functional asymmetries in ON and OFF ganglion cells of primate retina
- PMID: 11923439
- PMCID: PMC6758315
- DOI: 10.1523/JNEUROSCI.22-07-02737.2002
Functional asymmetries in ON and OFF ganglion cells of primate retina
Erratum in
- J Neurosci 2002 Oct 1;22(19):1a
Abstract
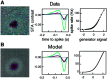
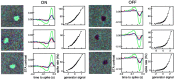
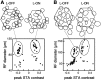
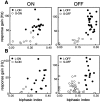
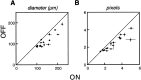
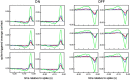
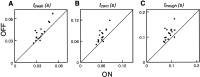
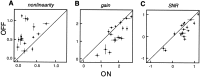
Functional asymmetries in the ON and OFF pathways of the primate visual system were examined using simultaneous multi-electrode recordings from dozens of retinal ganglion cells (RGCs) in vitro. Light responses of RGCs were characterized using white noise stimulation. Two distinct functional types of cells frequently encountered, one ON and one OFF, had non-opponent spectral sensitivity, relatively high response gain, transient light responses, and large receptive fields (RFs) that tiled the region of retina recorded, suggesting that they belonged to the same morphological cell class, most likely parasol. Three principal functional asymmetries were observed. (1) Receptive fields of ON cells were 20% larger in diameter than those of OFF cells, resulting in higher full-field sensitivity. (2) ON cells had faster response kinetics than OFF cells, with a 10-20% shorter time to peak, trough and zero crossing in the biphasic temporal impulse response. (3) ON cells had more nearly linear light responses and were capable of signaling decrements, whereas OFF cells had more strongly rectifying responses that provided little information about increments. These findings suggest specific mechanistic asymmetries in retinal ON and OFF circuits and differences in visual performance on the basis of the activity of ON and OFF parasol cells.
Figures



References
-
- Benardete EA, Kaplan E. The receptive field of the primate P retinal ganglion cell, I: Linear dynamics. Vis Neurosci. 1997;14:169–185. - PubMed
-
- Benardete EA, Kaplan E. The dynamics of primate M retinal ganglion cells. Vis Neurosci. 1999;16:355–368. - PubMed
-
- Bowen RW, Pokorny J, Smith VC. Sawtooth contrast sensitivity: decrements have the edge. Vision Res. 1989;29:1501–1509. - PubMed
-
- Chichilnisky EJ. A simple white noise analysis of neuronal light responses. Network: Comput Neural Syst. 2001;12:199–213. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
