Microtubule-associated protein 1A is a modifier of tubby hearing (moth1)
- PMID: 11925566
- PMCID: PMC2862212
- DOI: 10.1038/ng838
Microtubule-associated protein 1A is a modifier of tubby hearing (moth1)
Abstract
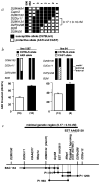
Once a mutation in the gene tub was identified as the cause of obesity, retinal degeneration and hearing loss in tubby mice, it became increasingly evident that the members of the tub gene family (tulps) influence maintenance and function of the neuronal cell lineage. Suggested molecular functions of tubby-like proteins include roles in vesicular trafficking, mediation of insulin signaling and gene transcription. The mechanisms through which tub functions in neurons, however, have yet to be elucidated. Here we report the positional cloning of an auditory quantitative trait locus (QTL), the modifier of tubby hearing 1 gene (moth1), whose wildtype alleles from strains AKR/J, CAST/Ei and 129P2/OlaHsd protect tubby mice from hearing loss. Through a transgenic rescue experiment, we verified that sequence polymorphisms in the neuron-specific microtubule-associated protein 1a gene (Mtap1a) observed in the susceptible strain C57BL/6J (B6) are crucial for the hearing-loss phenotype. We also show that these polymorphisms change the binding efficiency of MTAP1A to postsynaptic density molecule 95 (PSD95), a core component in the cytoarchitecture of synapses. This indicates that at least some of the observed polymorphisms are functionally important and that the hearing loss in C57BL/6J-tub/tub (B6-tub/tub) mice may be caused by impaired protein interactions involving MTAP1A. We therefore propose that tub may be associated with synaptic function in neuronal cells.
Figures


Comment in
-
Putting tubby on the MAP.Nat Genet. 2002 Apr;30(4):347-8. doi: 10.1038/ng0402-347. Nat Genet. 2002. PMID: 11925554 No abstract available.
References
-
- Noben-Trauth K, Naggert JK, North MA, Nishina PM. A candidate gene for the mouse mutation tubby. Nature. 1996;380:534–538. - PubMed
-
- Kleyn PW, et al. Identification and characterization of the mouse obesity gene tubby: a member of a novel gene family. Cell. 1996;85:281–290. - PubMed
-
- Ikeda S, et al. Cell specific expression of tubby gene family members in the retina. Invest Ophthalmol Vis Sci. 1999;40:2706–2712. - PubMed
-
- Hagstrom SA, Duyao M, North MA, Li T. Retinal degeneration in tulp1−/− mice: vesicular accumulation in the interphotoreceptor matrix. Invest Ophthalmol Vis Sci. 1999;40:2795–2802. - PubMed
-
- Ikeda S, et al. Retinal degeneration but not obesity is observed in null mutants of the tubby-like protein 1 gene. Hum Mol Genet. 2000;22:155–163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

