Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1
- PMID: 11927605
- PMCID: PMC2173255
- DOI: 10.1083/jcb.200108089
Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1
Abstract
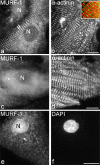
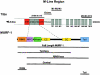
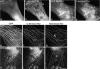
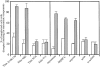
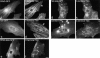
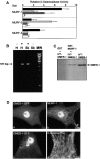
The COOH-terminal A168-170 region of the giant sarcomeric protein titin interacts with muscle-specific RING finger-1 (MURF-1). To investigate the functional significance of this interaction, we expressed green fluorescent protein fusion constructs encoding defined fragments of titin's M-line region and MURF-1 in cardiac myocytes. Upon expression of MURF-1 or its central region (containing its titin-binding site), the integrity of titin's M-line region was dramatically disrupted. Disruption of titin's M-line region also resulted in a perturbation of thick filament components, but, surprisingly, not of the NH2-terminal or I-band regions of titin, the Z-lines, or the thin filaments. This specific phenotype also was caused by the expression of titin A168-170. These data suggest that the interaction of titin with MURF-1 is important for the stability of the sarcomeric M-line region.MURF-1 also binds to ubiquitin-conjugating enzyme-9 and isopeptidase T-3, enzymes involved in small ubiquitin-related modifier-mediated nuclear import, and with glucocorticoid modulatory element binding protein-1 (GMEB-1), a transcriptional regulator. Consistent with our in vitro binding data implicating MURF-1 with nuclear functions, endogenous MURF-1 also was detected in the nuclei of some myocytes. The dual interactions of MURF-1 with titin and GMEB-1 may link myofibril signaling pathways (perhaps including titin's kinase domain) with muscle gene expression.
Figures



References
-
- Bang, M.L., T. Centner, F. Fornoff, A.J. Geach, M. Gotthardt, M. McNabb, C.C. Witt, D. Labeit, C.C. Gregorio, H. Granzier, and S. Labeit. 2001. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ. Res. 89:1065–1072. - PubMed
-
- Bodine, S.C., E. Latres, S. Baumhuerer, V.K. Lai, L. Nunez, B.A. Clarke, W.T. Poueymirou, F.J. Panaro, E. Na, K. Dharmarajan, Z.Q. Pan, D.M. Valenzuela, T.M. DeChiara, T.N. Stirr, G.D. Yancopoulos, and D.J. Glass. 2001. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 294:1704–1708. - PubMed
-
- Borden, K.L. 2000. RING domains: master builders of molecular scaffolds? J. Mol. Biol. 295:1103–1112. - PubMed
-
- Centner, T., F. Fougerousse, A. Freiburg, C. Witt, J.S. Beckmann, H. Granzier, K. Trombitas, C.C. Gregorio, and S. Labeit. 2000. Molecular tools for the study of titin's differential expression. Adv. Exp. Med. Biol. 481:35–49. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

