Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice
- PMID: 11927606
- PMCID: PMC2173262
- DOI: 10.1083/jcb.200108071
Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice
Abstract
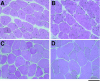
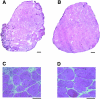
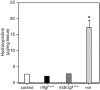
Duchenne muscular dystrophy is an X-linked degenerative disorder of muscle caused by the absence of the protein dystrophin. A major consequence of muscular dystrophy is that the normal regenerative capacity of skeletal muscle cannot compensate for increased susceptibility to damage, leading to repetitive cycles of degeneration-regeneration and ultimately resulting in the replacement of muscle fibers with fibrotic tissue. Because insulin-like growth factor I (IGF-I) has been shown to enhance muscle regeneration and protein synthetic pathways, we asked whether high levels of muscle-specific expression of IGF-I in mdx muscle could preserve muscle function in the diseased state. In transgenic mdx mice expressing mIgf-I (mdx:mIgf+/+), we showed that muscle mass increased by at least 40% leading to similar increases in force generation in extensor digitorum longus muscles compared with those from mdx mice. Diaphragms of transgenic mdx:mIgf+/+ exhibited significant hypertrophy and hyperplasia at all ages observed. Furthermore, the IGF-I expression significantly reduced the amount of fibrosis normally observed in diaphragms from aged mdx mice. Decreased myonecrosis was also observed in diaphragms and quadriceps from mdx:mIgf+/+ mice when compared with age-matched mdx animals. Finally, signaling pathways associated with muscle regeneration and protection against apoptosis were significantly elevated. These results suggest that a combination of promoting muscle regenerative capacity and preventing muscle necrosis could be an effective treatment for the secondary symptoms caused by the primary loss of dystrophin.
Figures












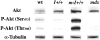
References
-
- Adams, G.R., and S.A. McCue. 1998. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J. Appl. Physiol. 84:1716–1722. - PubMed
-
- Alessi, D.R., and P. Cohen. 1998. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 8:55–62. - PubMed
-
- Allen, R.E., S.M. Sheehan, R.G. Taylor, T.L. Kendall, and G.M. Rice. 1995. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J. Cell. Physiol. 165:307–312. - PubMed
-
- Anderson, J.E., W.K. Ovalle, and B.H. Bressler. 1987. Electron microscopic and autoradiographic characterization of hindlimb muscle regeneration in the mdx mouse. Anat. Rec. 219:243–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

