Regulation of herpes simplex virus gamma(1)34.5 expression and oncolysis of diffuse liver metastases by Myb34.5
- PMID: 11927614
- PMCID: PMC150923
- DOI: 10.1172/JCI10623
Regulation of herpes simplex virus gamma(1)34.5 expression and oncolysis of diffuse liver metastases by Myb34.5
Abstract
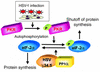
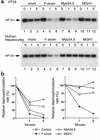
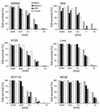
Myb34.5 is a herpes simplex virus 1 (HSV-1) mutant deleted in the gene for ribonucleotide reductase (ICP6). It also carries a version of gamma(1)34.5 (a viral gene product that promotes the dephosphorylation of eIF-2alpha) that is under control of the E2F-responsive cellular B-myb promoter, rather than of its endogenous promoter. Myb34.5 replication in tumor cells results in their destruction (oncolysis). gamma(1)34.5 expression by HSV-1 subverts an important cell defense mechanism against viral replication by preventing shutoff of protein synthesis after viral infection. Infection of colon carcinoma cells with Myb34.5 results in greater eIF-2alpha dephosphorylation and viral replication compared with infection with HSV-1 mutants completely defective in gamma(1)34.5 expression. In contrast, infection of normal hepatocytes with Myb34.5 results in low levels of eIF-2alpha dephosphorylation and viral replication that are similar to those observed with HSV-1 mutants completely defective in gamma(1)34.5 and ICP6. When administered intravascularly into mice with diffuse liver metastases, Myb34.5 has greater antineoplastic activity than HSV-1 mutants with completely defective gamma(1)34.5 expression and more restricted biodistribution compared with HSV-1 mutants with wild-type gamma(1)34.5 expression. Myb34.5 displays reduced virulence and toxicity compared to HSV-1 mutants with wild-type gamma(1)34.5 expression. Portal venous administration of Myb34.5 significantly reduces liver tumor burden in and prolongs the life of mice with diffuse liver metastases. Preexisting Ab's to HSV-1 do not reduce the antitumor efficacy of Myb34.5 in vivo.
Figures
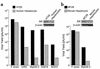


References
-
- Rosenberg SA, et al. Human gene marker/therapy clinical protocols. Hum Gene Ther. 2000;11:919–979. - PubMed
-
- Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21–39. - PubMed
-
- Bischoff JR, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. - PubMed
-
- Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging. Nat Med. 1998;4:1341–1342. - PubMed
-
- Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

