GAD65-reactive T cells are activated in patients with autoimmune type 1a diabetes
- PMID: 11927616
- PMCID: PMC150925
- DOI: 10.1172/JCI14114
GAD65-reactive T cells are activated in patients with autoimmune type 1a diabetes
Erratum in
- J Clin Invest 2002 Jun;109(11):1511
Abstract
Insulin-dependent type 1 diabetes is an autoimmune disease mediated by T lymphocytes recognizing pancreatic islet cell antigens. Glutamic acid decarboxylase 65 (GAD65) appears to be an important autoantigen in the disease. However, T cells from both patients with type 1 diabetes and healthy subjects vigorously proliferate in response to GAD65 stimulation ex vivo, leading us to postulate that the critical event in the onset of human diabetes is the activation of autoreactive T cells. Thus, we investigated whether GAD65-reactive T cells in patients with diabetes functioned as previously activated memory T cells, no longer requiring a second, costimulatory signal for clonal expansion. We found that in patients with new-onset type 1 diabetes, GAD65-reactive T cells were strikingly less dependent on CD28 and B7-1 costimulation to enter into cell cycle and proliferate than were equivalent cells derived from healthy controls. We hypothesize that these autoreactive T cells have been activated in vivo and have differentiated into memory cells, suggesting a pathogenic role in type 1 diabetes. In addition, we observed different effects with selective blockade of either B7-1 or B7-2 molecules; B7-1 appears to deliver a negative signal by engaging CTLA-4, while B7-2 engagement of CD28 upregulates T cell proliferation and cytokine secretion.
Figures

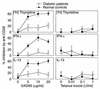


Comment in
-
Controlling the controls: GAD65 autoreactive T cells in type 1 diabetes.J Clin Invest. 2002 Apr;109(7):869-70. doi: 10.1172/JCI15381. J Clin Invest. 2002. PMID: 11927613 Free PMC article. Review. No abstract available.
References
-
- Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. - PubMed
-
- Roep BO, De Vries RR. T-lymphocytes and the pathogenesis of type 1 (insulin-dependent) diabetes mellitus. Eur J Clin Invest. 1992;22:697–711. - PubMed
-
- Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. - PubMed
-
- Stiller CR, et al. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science. 1984;223:1362–1367. - PubMed
-
- Bougneres PF, et al. Factors associated with early remission of type I diabetes in children treated with cyclosporine. N Engl J Med. 1988;318:663–670. - PubMed

