Calcium-induced transitions between the spontaneous miniature outward and the transient outward currents in retinal amacrine cells
- PMID: 11929887
- PMCID: PMC2311395
- DOI: 10.1085/jgp.20028479
Calcium-induced transitions between the spontaneous miniature outward and the transient outward currents in retinal amacrine cells
Abstract
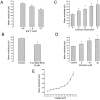
Spontaneous miniature outward currents (SMOCs) occur in a subset of retinal amacrine cells at membrane potentials between -60 and -40 mV. At more depolarized potentials, a transient outward current (I(to)) appears and SMOCs disappear. Both SMOCs and the I(to) are K(+) currents carried by BK channels. They both arise from Ca(2+) influx through high voltage-activated (HVA) Ca(2+) channels, which stimulates release of internal Ca(2+) from caffeine- and ryanodine-sensitive stores. An increase in Ca(2+) influx resulted in an increase in SMOC frequency, but also led to a decline in SMOC mean amplitude. This reduction showed a temporal dependence: the effect being greater in the latter part of a voltage step. Thus, Ca(2+) influx, although required to generate SMOCs, also produced a negative modulation of their amplitudes. Increasing Ca(2+) influx also led to a decline in the first latency to SMOC occurrence. A combination of these effects resulted in the disappearance of SMOCs, along with the concomitant appearance of the I(to) at high levels of Ca(2+) influx. Therefore, low levels of Ca(2+) influx, arising from low levels of activation of the HVA Ca(2+) channels, produce randomly occurring SMOCs within the range of -60 to -40 mV. Further depolarization leads to greater activation of the HVA Ca(2+) channels, larger Ca(2+) influx, and the disappearance of discontinuous SMOCs, along with the appearance of the I(to). Based on their characteristics, SMOCs in retinal neurons may function as synaptic noise suppressors at quiescent glutamatergic synapses.
Figures





Similar articles
-
Mechanism of generation of spontaneous miniature outward currents (SMOCs) in retinal amacrine cells.J Gen Physiol. 2002 Apr;119(4):355-72. doi: 10.1085/jgp.20028478. J Gen Physiol. 2002. PMID: 11929886 Free PMC article.
-
Ca(2+)-induced Ca(2+) release activates spontaneous miniature outward currents (SMOCs) in parasympathetic cardiac neurons.J Neurophysiol. 1999 Aug;82(2):540-50. doi: 10.1152/jn.1999.82.2.540. J Neurophysiol. 1999. PMID: 10444654
-
Number of K(Ca) channels underlying spontaneous miniature outward currents (SMOCs) in mudpuppy cardiac neurons.J Neurophysiol. 2001 Jan;85(1):54-60. doi: 10.1152/jn.2001.85.1.54. J Neurophysiol. 2001. PMID: 11152705
-
Effects of intracellular stores and extracellular Ca(2+) on Ca(2+)-activated K(+) currents in mature mouse inner hair cells.J Physiol. 2004 Jun 1;557(Pt 2):613-33. doi: 10.1113/jphysiol.2003.060137. Epub 2004 Apr 2. J Physiol. 2004. PMID: 15064328 Free PMC article.
-
Pharmacological and functional properties of voltage-independent Ca2+ channels.Cell Calcium. 1996 Apr;19(4):269-79. doi: 10.1016/s0143-4160(96)90068-8. Cell Calcium. 1996. PMID: 8983848 Review.
Cited by
-
Ryanodine-induced oppositely directed changes in frequency of spontaneous mediator secretion.Dokl Biol Sci. 2004 Jul-Aug;397:265-8. doi: 10.1023/b:dobs.0000039687.80974.09. Dokl Biol Sci. 2004. PMID: 15508569 No abstract available.
-
BK Channels in the Central Nervous System.Int Rev Neurobiol. 2016;128:281-342. doi: 10.1016/bs.irn.2016.04.001. Epub 2016 May 13. Int Rev Neurobiol. 2016. PMID: 27238267 Free PMC article. Review.
-
Mechanism of generation of spontaneous miniature outward currents (SMOCs) in retinal amacrine cells.J Gen Physiol. 2002 Apr;119(4):355-72. doi: 10.1085/jgp.20028478. J Gen Physiol. 2002. PMID: 11929886 Free PMC article.
-
Anatomical and neurochemical characterization of dopaminergic interplexiform processes in mouse and rat retinas.J Comp Neurol. 2008 Sep 10;510(2):158-74. doi: 10.1002/cne.21784. J Comp Neurol. 2008. PMID: 18615559 Free PMC article.
-
GABAB receptors enhance excitatory responses in isolated rat retinal ganglion cells.J Physiol. 2016 Oct 1;594(19):5543-54. doi: 10.1113/JP272374. Epub 2016 Jun 14. J Physiol. 2016. PMID: 27112134 Free PMC article.
References
-
- Bean, B.P. 1989. Classes of calcium channels in vertebrate cells. Annu. Rev. Physiol. 51:367–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

