Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A
- PMID: 11929983
- PMCID: PMC122755
- DOI: 10.1073/pnas.082100499
Catalysis of cis/trans isomerization in native HIV-1 capsid by human cyclophilin A
Abstract
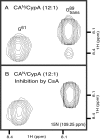
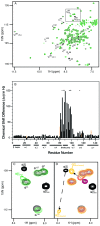
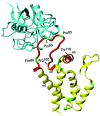
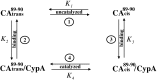
Packaging of cyclophilin A (CypA) into HIV-1 virions is essential for efficient replication; however, the reason for this is unknown. Incorporation is mediated through binding to the Gly-89-Pro-90 peptide bond of the N-terminal domain of HIV-1 capsid (CA(N)). Despite the fact that CypA is a peptidyl-prolyl cis/trans isomerase, catalytic activity on CA(N) has not been observed previously. We show here, using NMR exchange spectroscopy, that CypA does not only bind to CA(N) but also catalyzes efficiently the cis/trans isomerization of the Gly-89-Pro-90 peptide bond. In addition, conformational changes in CA(N) distal to the CypA binding loop are observed on CypA binding and catalysis. The results provide experimental evidence for efficient CypA catalysis on a natively folded and biologically relevant protein substrate.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

