FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction
- PMID: 11930000
- PMCID: PMC123646
- DOI: 10.1073/pnas.261702698
FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction
Abstract
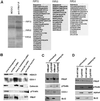
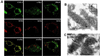
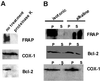
FKBP12-rapamycin associated protein (FRAP, also known as mTOR or RAFT) is the founding member of the phosphatidylinositol kinase-related kinase family and functions as a sensor of physiological signals that regulate cell growth. Signals integrated by FRAP include nutrients, cAMP levels, and osmotic stress, and cellular processes affected by FRAP include transcription, translation, and autophagy. The mechanisms underlying the integration of such diverse signals by FRAP are largely unknown. Recently, FRAP has been reported to be regulated by mitochondrial dysfunction and depletion of ATP levels. Here we show that exposure of cells to hyperosmotic conditions (and to glucose-deficient growth medium) results in rapid and reversible dissipation of the mitochondrial proton gradient. These results suggest that the ability of FRAP to mediate osmotic stress response (and glucose deprivation response) is by means of an intermediate mitochondrial dysfunction. We also show that in addition to cytosolic FRAP a large portion of FRAP associates with the mitochondrial outer membrane. The results support the existence of a stress-sensing module consisting of mitochondria and mitochondrial outer membrane-associated FRAP. This module allows the cell to integrate a variety of stress signals that affect mitochondrial function and regulate a growth checkpoint involving p70 S6 kinase.
Figures

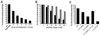

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

