Evolutionary conservation of chromosome territory arrangements in cell nuclei from higher primates
- PMID: 11930003
- PMCID: PMC123664
- DOI: 10.1073/pnas.072618599
Evolutionary conservation of chromosome territory arrangements in cell nuclei from higher primates
Abstract
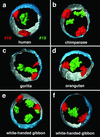
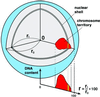
We demonstrate that the nuclear topological arrangement of chromosome territories (CTs) has been conserved during primate evolution over a period of about 30 million years. Recent evidence shows that the positioning of chromatin in human lymphocyte nuclei is correlated with gene density. For example, human chromosome 19 territories, which contain mainly gene-dense and early replicating chromatin, are located toward the nuclear center, whereas chromosome 18 territories, which consist mainly of gene-poor and later replicating chromatin, is located close to the nuclear border. In this study, we subjected seven different primate species to comparative analysis of the radial distribution pattern of human chromosome 18- and 19-homologous chromatin by three-dimensional fluorescence in situ hybridization. Our data demonstrate that gene-density-correlated radial chromatin arrangements were conserved during higher-primate genome evolution, irrespective of the major karyotypic rearrangements that occurred in different phylogenetic lineages. The evolutionarily conserved positioning of homologous chromosomes or chromosome segments in related species supports evidence for a functionally relevant higher-order chromatin arrangement that is correlated with gene-density.
Figures
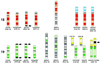



References
-
- Chevret E, Volpi E V, Sheer D. Cytogenet Cell Genet. 2000;90:13–21. - PubMed
-
- Cremer T, Cremer C. Nat Rev Genet. 2001;2:292–301. - PubMed
-
- Cremer T, Kreth G, Koester H, Fink R H A, Heintzmann R, Cremer M, Solovei I V, Zink D, Cremer C. Crit Rev Eukaryotic Gene Expression. 2000;12:179–212. - PubMed
-
- Lamond A I, Earnshaw W C. Science. 1998;280:547–553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

