Lung infections associated with cystic fibrosis
- PMID: 11932230
- PMCID: PMC118069
- DOI: 10.1128/CMR.15.2.194-222.2002
Lung infections associated with cystic fibrosis
Abstract
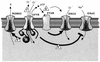
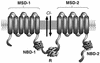
While originally characterized as a collection of related syndromes, cystic fibrosis (CF) is now recognized as a single disease whose diverse symptoms stem from the wide tissue distribution of the gene product that is defective in CF, the ion channel and regulator, cystic fibrosis transmembrane conductance regulator (CFTR). Defective CFTR protein impacts the function of the pancreas and alters the consistency of mucosal secretions. The latter of these effects probably plays an important role in the defective resistance of CF patients to many pathogens. As the modalities of CF research have changed over the decades from empirical histological studies to include biophysical measurements of CFTR function, the clinical management of this disease has similarly evolved to effectively address the ever-changing spectrum of CF-related infectious diseases. These factors have led to the successful management of many CF-related infections with the notable exception of chronic lung infection with the gram-negative bacterium Pseudomonas aeruginosa. The virulence of P. aeruginosa stems from multiple bacterial attributes, including antibiotic resistance, the ability to utilize quorum-sensing signals to form biofilms, the destructive potential of a multitude of its microbial toxins, and the ability to acquire a mucoid phenotype, which renders this microbe resistant to both the innate and acquired immunologic defenses of the host.
Figures

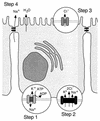


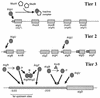
 , constitutive (σ70-like) promoter;
, constitutive (σ70-like) promoter;  , heat shock (σ54-like) promoter;
, heat shock (σ54-like) promoter;  , transcriptional activator;
, transcriptional activator;  , transcriptional inhibitor;
, transcriptional inhibitor;  , structural gene; ➞, positive regulation; →, posttranslational modification;
, structural gene; ➞, positive regulation; →, posttranslational modification;  , kinase; -P, phosphate.
, kinase; -P, phosphate.References
-
- Reference deleted.
-
- Allan, J. D., A. Mason, and A. D. Moss. 1973. Nutritional supplementation in treatment of cystic fibrosis of the pancreas. Am. J. Dis. Child. 126:22-26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

