Dracunculiasis (Guinea worm disease) and the eradication initiative
- PMID: 11932231
- PMCID: PMC118073
- DOI: 10.1128/CMR.15.2.223-246.2002
Dracunculiasis (Guinea worm disease) and the eradication initiative
Abstract
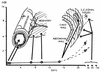
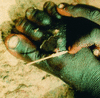
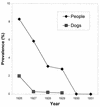
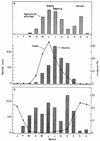
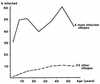
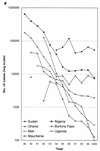
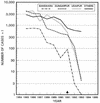
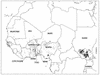
Dracunculiasis, also known as guinea worm disease, is caused by the large female of the nematode Dracunculus medinensis, which emerges painfully and slowly from the skin, usually on the lower limbs. The disease can infect animals, and sustainable animal cycles occur in North America and Central Asia but do not act as reservoirs of human infection. The disease is endemic across the Sahel belt of Africa from Mauritania to Ethiopia, having been eliminated from Asia and some African countries. It has a significant socioeconomic impact because of the temporary disability that it causes. Dracunculiasis is exclusively caught from drinking water, usually from ponds. A campaign to eradicate the disease was launched in the 1980s and has made significant progress. The strategy of the campaign is discussed, including water supply, health education, case management, and vector control. Current issues including the integration of the campaign into primary health care and the mapping of cases by using geographic information systems are also considered. Finally, some lessons for other disease control and eradication programs are outlined.
Figures






References
-
- Abolarin, M. O. 1981. Guinea worm infection in a Nigerian village. Trop. Geogr. Med 33:83-88. - PubMed
-
- Adekolu-John, E. A. 1983. The impact of lake creation on guinea-worm transmission in Nigeria on the Eastern side of Kainji Lake. Int. J. Parasitol 13:427-432. - PubMed
-
- Adeyeba, O. A. 1985. Secondary infection in dracunculiasis: bacteria and morbidity. Int. J. Zoonoses 12:147-149. - PubMed
-
- Adeyeba, O. A., and O. O. Kale. 1991. Epidemiology of dracunculiasis and its socio-economic impact in a village in south-west Nigeria. West Afr. J. Med 10:208-215. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

