Innate immunity to Mycobacterium tuberculosis
- PMID: 11932234
- PMCID: PMC118070
- DOI: 10.1128/CMR.15.2.294-309.2002
Innate immunity to Mycobacterium tuberculosis
Abstract
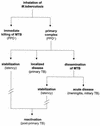
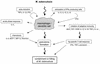
The different manifestations of infection with Mycobacterium tuberculosis reflect the balance between the bacillus and host defense mechanisms. Traditionally, protective immunity to tuberculosis has been ascribed to T-cell-mediated immunity, with CD4(+) T cells playing a crucial role. Recent immunological and genetic studies support the long-standing notion that innate immunity is also relevant in tuberculosis. In this review, emphasis is on these natural, innate host defense mechanisms, referring to experimental data (e.g., studies in gene knockout mice) and epidemiological, immunological, and genetic studies in human tuberculosis. The first step in the innate host defense is cellular uptake of M. tuberculosis, which involves different cellular receptors and humoral factors. Toll-like receptors seem to play a crucial role in immune recognition of M. tuberculosis, which is the next step. The subsequent inflammatory response is regulated by production of pro- and anti-inflammatory cytokines and chemokines. Different natural effector mechanisms for killing of M. tuberculosis have now been identified. Finally, the innate host response is necessary for induction of adaptive immunity to M. tuberculosis. These basic mechanisms augment our understanding of disease pathogenesis and clinical course and will be of help in designing adjunctive treatment strategies.
Figures


References
-
- Abbas, A. K., K. M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature 383:787-793. - PubMed
-
- Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. - PubMed
-
- Altare, F., A. Durandy, D. Lammas, J. Emile, S. Lamhamedi, F. Le Deist, P. Drysdale, E. Jouanguy, R. Doffinger, F. Bernaudin, O. Jeppsson, J. A. Gollob, E. Meinl, A. W. Segal, A. Fischer, D. Kumararatne, and J. L. Casanova. 1998. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science 280:1432-1435. - PubMed
-
- Altare, F., A. Ensser, A. Breiman, J. Reichenbach, J. E. Baghdadi, A. Fischer, J. F. Emile, J. L. Gaillard, E. Meinl, and J. L. Casanova. 2001. Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J. Infect. Dis. 184:231-236. - PubMed
-
- Altare, F., D. Lammas, P. Revy, E. Jouanguy, R. Doffinger, S. Lamhamedi, P. Drysdale, D. Scheel Toellner, J. Girdlestone, P. Darbyshire, M. Wadhwa, H. Dockrell, M. Salmon, A. Fischer, A. Durandy, J. L. Casanova, and D. S. Kumararatne. 1998. Inherited interleukin 12 deficiency in a child with bacille Calmette-Guerin and Salmonella enteritidis disseminated infection. J. Clin. Investig. 102:2035-2040. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

