Angiogenic effects of extracellular human immunodeficiency virus type 1 Tat protein and its role in the pathogenesis of AIDS-associated Kaposi's sarcoma
- PMID: 11932235
- PMCID: PMC118071
- DOI: 10.1128/CMR.15.2.310-326.2002
Angiogenic effects of extracellular human immunodeficiency virus type 1 Tat protein and its role in the pathogenesis of AIDS-associated Kaposi's sarcoma
Abstract
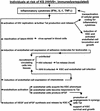
The Tat protein of human immunodeficiency virus (HIV) type 1 is a transactivator of viral gene expression that is required for virus replication and spread. Moreover, Tat is released by acutely HIV-infected cells via a leaderless secretory pathway and in a biologically active form that exerts effects on both HIV-infected and uninfected cells from different organs and systems. This review focuses on the activities of extracellular Tat protein on endothelial cells, on angiogenesis, and on the pathogenesis of AIDS-associated angioproliferative diseases such as Kaposi's sarcoma. In particular, we discuss results from different groups indicating that Tat mimics the proangiogenic activities of extracellular matrix molecules and that it enhances the effects of angiogenic factors.
Figures



References
-
- Aboulafia, D., S. A. Miles, S. R. Saks, and R. T. Mitsuyasu. 1989. Intravenous recombinant tumor necrosis factor in the treatment of AIDS-related Kaposi's sarcoma. J. Acquir. Immune Defic. Syndr. 2:54-58. - PubMed
-
- Albini, A., R. Soldi, D. Giunciuglio, E. Giraudo, R. Benelli, L. Primo, D. Noonan, M. Salio, G. Camussi, W. Rockl, and F. Bussolino. 1996. The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 2:1371-1375. - PubMed
-
- Albini, A., R. Benelli, M. Presta, M. Rusnati, M. Ziche, A. Rubartelli, G. Paglialunga, F. Bussolino, and D. Noonan. 1996. HIV-1 tat protein is a heparin binding angiogenic growth factor. Oncogene 12:289-297. - PubMed
-
- Albini, A., R. Benelli, D. Giunciuglio, T. Cai, G. Mariani, S. Ferrini, and D. Noonan. 1998. Identification of a novel domain of HIV tat involved in monocyte chemotaxis. J. Biol. Chem. 273:15895-15900. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

