Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion
- PMID: 11932406
- PMCID: PMC155071
- DOI: 10.1128/jvi.76.9.4390-4400.2002
Human herpesvirus 8 glycoprotein B (gB), gH, and gL can mediate cell fusion
Abstract
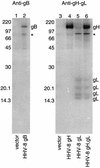
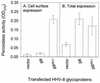
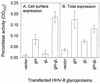
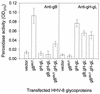
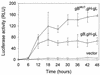
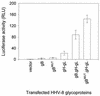
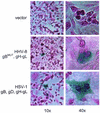
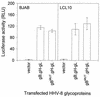
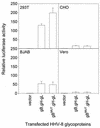
Herpesvirus entry into cells and herpesvirus-induced cell fusion are related processes in that virus penetration proceeds by fusion of the viral envelope and cell membrane. To characterize the human herpesvirus 8 (HHV-8) glycoproteins that can mediate cell fusion, a luciferase reporter gene activation assay was used. Chinese hamster ovary (CHO) cells expressing the HHV-8 glycoproteins of interest along with a luciferase reporter gene under the control of the T7 promoter were cocultivated with human cells transfected with T7 RNA polymerase. Because HHV-8 glycoprotein B (gB) expressed in CHO cells localizes to the perinuclear region, a truncated form of gB (designated gB(MUT)) that lacks putative endocytosis signals was constructed by deletion of the distal 58 amino acids of the cytoplasmic tail. HHV-8 gB(MUT) was expressed efficiently on the surface of CHO cells. HHV-8 gB, gH, and gL could mediate the fusion of CHO cells with two different human cell types, embryonic kidney cells and B lymphocytes. Substituting gB(MUT) for gB significantly enhanced the fusion of CHO cells with human embryonic kidney cells but not B lymphocytes. Thus, two human cell types known to be susceptible to HHV-8 entry were also suitable targets for cell fusion induced by HHV-8 gB, gH, and gL. For human embryonic kidney cells and B cells at least, optimal fusion was noted with the expression of all three HHV-8 glycoproteins.
Figures

Similar articles
-
Human herpesvirus 8 envelope glycoprotein B mediates cell adhesion via its RGD sequence.J Virol. 2003 Mar;77(5):3131-47. doi: 10.1128/jvi.77.5.3131-3147.2003. J Virol. 2003. PMID: 12584338 Free PMC article.
-
Human herpesvirus-8 glycoprotein B interacts with Epstein-Barr virus (EBV) glycoprotein 110 but fails to complement the infectivity of EBV mutants.Virology. 1998 Nov 25;251(2):402-13. doi: 10.1006/viro.1998.9412. Virology. 1998. PMID: 9837804
-
Functional Relevance of the N-Terminal Domain of Pseudorabies Virus Envelope Glycoprotein H and Its Interaction with Glycoprotein L.J Virol. 2017 Apr 13;91(9):e00061-17. doi: 10.1128/JVI.00061-17. Print 2017 May 1. J Virol. 2017. PMID: 28228592 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements.J Virol. 2004 Apr;78(8):4207-23. doi: 10.1128/jvi.78.8.4207-4223.2004. J Virol. 2004. PMID: 15047836 Free PMC article.
-
Herpesvirus entry: an update.J Virol. 2003 Oct;77(19):10179-85. doi: 10.1128/jvi.77.19.10179-10185.2003. J Virol. 2003. PMID: 12970403 Free PMC article. Review. No abstract available.
Cited by
-
Modulation of Epstein-Barr virus glycoprotein B (gB) fusion activity by the gB cytoplasmic tail domain.mBio. 2013 Jan 22;4(1):e00571-12. doi: 10.1128/mBio.00571-12. mBio. 2013. PMID: 23341550 Free PMC article.
-
KSHV: pathways to tumorigenesis and persistent infection.Adv Virus Res. 2014;88:111-59. doi: 10.1016/B978-0-12-800098-4.00002-7. Adv Virus Res. 2014. PMID: 24373311 Free PMC article. Review.
-
Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) infection of human fibroblast cells occurs through endocytosis.J Virol. 2003 Jul;77(14):7978-90. doi: 10.1128/jvi.77.14.7978-7990.2003. J Virol. 2003. PMID: 12829837 Free PMC article.
-
Structural proteins of Kaposi's sarcoma-associated herpesvirus antagonize p53-mediated apoptosis.Oncogene. 2015 Jan 29;34(5):639-49. doi: 10.1038/onc.2013.595. Epub 2014 Jan 27. Oncogene. 2015. PMID: 24469037
-
The Neutralizing Linear Epitope of Human Herpesvirus 6A Glycoprotein B Does Not Affect Virus Infectivity.J Virol. 2018 Feb 12;92(5):e02074-17. doi: 10.1128/JVI.02074-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29212944 Free PMC article.
References
-
- Akula, S. M., N. P. Pramod, F.-Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. - PubMed
-
- Akula, S. M., F.-Z. Wang, J. Vieira, and B. Chandran. 2001. Human herpesvirus 8 interaction with target cells involves heparan sulfate. Virology 282:245-255. - PubMed
-
- Ambroziak, J. A., D. J. Blackbourn, B. G. Herndier, R. G. Glogau, J. H. Gullett, A. R. McDonald, E. T. Lennette, and J. A. Levy. 1995. Herpes-like sequences in HIV-infected and uninfected Kaposi's sarcoma patients. Science 268:582-583. - PubMed
-
- Baghian, A., M. Luftig, J. B. Black, Y.-X. Meng, C.-P. Pau, T. Voss, P. E. Pellett, and K. G. Kousoulas. 2000. Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18-25. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

