Role of recycling endosomes and lysosomes in dynein-dependent entry of canine parvovirus
- PMID: 11932407
- PMCID: PMC155078
- DOI: 10.1128/jvi.76.9.4401-4411.2002
Role of recycling endosomes and lysosomes in dynein-dependent entry of canine parvovirus
Abstract
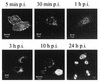
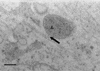
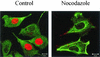
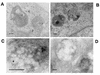
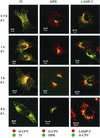
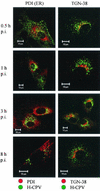
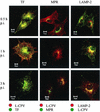
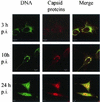
Canine parvovirus (CPV) is a nonenveloped virus with a 5-kb single-stranded DNA genome. Lysosomotropic agents and low temperature are known to prevent CPV infection, indicating that the virus enters its host cells by endocytosis and requires an acidic intracellular compartment for penetration into the cytoplasm. After escape from the endocytotic vesicles, CPV is transported to the nucleus for replication. In the present study the intracellular entry pathway of the canine parvovirus in NLFK (Nordisk Laboratory feline kidney) cells was studied. After clustering in clathrin-coated pits and being taken up in coated vesicles, CPV colocalized with coendocytosed transferrin in endosomes resembling recycling endosomes. Later, CPV was found to enter, via late endosomes, a perinuclear vesicular compartment, where it colocalized with lysosomal markers. There was no indication of CPV entry into the trans-Golgi or the endoplasmic reticulum. Similar results were obtained both with full and with empty capsids. The data thus suggest that CPV or its DNA was released from the lysosomal compartment to the cytoplasm to be then transported to the nucleus. Electron microscopy analysis revealed endosomal vesicles containing CPV to be associated with microtubules. In the presence of nocodazole, a microtubule-disrupting drug, CPV entry was blocked and the virus was found in peripheral vesicles. Thus, some step(s) of the entry process were dependent on microtubules. Microinjection of antibodies to dynein caused CPV to remain in pericellular vesicles. This suggests an important role for the motor protein dynein in transporting vesicles containing CPV along the microtubule network.
Figures



Similar articles
-
Intracellular route of canine parvovirus entry.J Virol. 1998 Jan;72(1):802-6. doi: 10.1128/JVI.72.1.802-806.1998. J Virol. 1998. PMID: 9420290 Free PMC article.
-
Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking.J Virol. 2000 Feb;74(4):1919-30. doi: 10.1128/jvi.74.4.1919-1930.2000. J Virol. 2000. PMID: 10644365 Free PMC article.
-
Release of canine parvovirus from endocytic vesicles.Virology. 2003 Nov 25;316(2):267-80. doi: 10.1016/j.virol.2003.08.031. Virology. 2003. PMID: 14644609
-
The complex ultrastructure of the endolysosomal system.Cold Spring Harb Perspect Biol. 2014 May 22;6(10):a016857. doi: 10.1101/cshperspect.a016857. Cold Spring Harb Perspect Biol. 2014. PMID: 24851870 Free PMC article. Review.
-
Tumor targeting using canine parvovirus nanoparticles.Curr Top Microbiol Immunol. 2009;327:123-41. doi: 10.1007/978-3-540-69379-6_6. Curr Top Microbiol Immunol. 2009. PMID: 19198573 Review.
Cited by
-
Oncolytic H-1 Parvovirus Enters Cancer Cells through Clathrin-Mediated Endocytosis.Viruses. 2020 Oct 21;12(10):1199. doi: 10.3390/v12101199. Viruses. 2020. PMID: 33096814 Free PMC article.
-
Effect of ATP binding and hydrolysis on dynamics of canine parvovirus NS1.J Virol. 2010 May;84(10):5391-403. doi: 10.1128/JVI.02221-09. Epub 2010 Mar 10. J Virol. 2010. PMID: 20219935 Free PMC article.
-
Mechanisms of cell death in canine parvovirus-infected cells provide intuitive insights to developing nanotools for medicine.Int J Nanomedicine. 2010 Aug 9;5:417-28. doi: 10.2147/ijn.s10579. Int J Nanomedicine. 2010. PMID: 20957163 Free PMC article.
-
Multiple pathways involved in porcine parvovirus cellular entry and trafficking toward the nucleus.J Virol. 2010 Aug;84(15):7782-92. doi: 10.1128/JVI.00479-10. Epub 2010 May 19. J Virol. 2010. PMID: 20484503 Free PMC article.
-
Mouse polyomavirus utilizes recycling endosomes for a traffic pathway independent of COPI vesicle transport.J Virol. 2003 Feb;77(3):1672-81. doi: 10.1128/jvi.77.3.1672-1681.2003. J Virol. 2003. PMID: 12525601 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

