Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines
- PMID: 11932419
- PMCID: PMC155070
- DOI: 10.1128/jvi.76.9.4536-4546.2002
Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines
Abstract
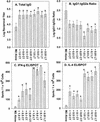
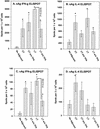
Two plasmid vectors encoding the A and B subunits of cholera toxin (CT) and two additional vectors encoding the A and B subunits of the Escherichia coli heat-labile enterotoxin (LT) were evaluated for their ability to serve as genetic adjuvants for particle-mediated DNA vaccines administered to the epidermis of laboratory animals. Both the CT and the LT vectors strongly augmented Th1 cytokine responses (gamma interferon [IFN-gamma]) to multiple viral antigens when codelivered with DNA vaccines. In addition, Th2 cytokine responses (interleukin 4 [IL-4]) were also augmented by both sets of vectors, with the effects of the LT vectors on IL-4 responses being more antigen dependent. The activities of both sets of vectors on antibody responses were antigen dependent and ranged from no effect to sharp reductions in the immunoglobulin G1 (IgG1)-to-IgG2a ratios. Overall, the LT vectors exhibited stronger adjuvant effects in terms of T-cell responses than did the CT vectors, and this was correlated with the induction of greater levels of cyclic AMP by the LT vectors following vector transfection into cultured cells. The adjuvant effects observed in vivo were due to the biological effects of the encoded proteins and not due to CpG motifs in the bacterial genes. Interestingly, the individual LT A and B subunit vectors exhibited partial adjuvant activity that was strongly influenced by the presence or absence of signal peptide coding sequences directing the encoded subunit to either intracellular or extracellular locations. Particle-mediated delivery of either the CT or LT adjuvant vectors in rodents and domestic pigs was well tolerated, suggesting that bacterial toxin-based genetic adjuvants may be a safe and effective strategy to enhance the potency of both prophylactic and therapeutic DNA vaccines for the induction of strong cellular immunity.
Figures

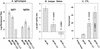

References
-
- Aderem, A., and D. A. Hume. 2000. How do you see CG? Cell 103:993-996. - PubMed
-
- Bauer, C. M., L. H. Pinto, T. A. Cross, and R. A. Lamb. 1999. The influenza virus M2 ion channel protein: probing the structure of the transmembrane domain in intact cells by using engineered disulfide cross-linking. Virology 254:196-209. - PubMed
-
- Boyer, J. D., A. D. Cohen, S. Vogt, K. Schumann, B. Nath, L. Ahn, K. Lacy, M. L. Bagarazzi, T. J. Higgins, Y. Baine, R. B. Ciccarelli, R. S. Ginsberg, R. R. MacGregor, and D. B. Weiner. 2000. Vaccination of seronegative volunteers with a human immunodeficiency virus type 1 env/rev DNA vaccine induces antigen-specific proliferation and lymphocyte production of beta-chemokines. J. Infect. Dis. 181:476-483. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

