The oncogenic protein kinase Tpl-2/Cot contributes to Epstein-Barr virus-encoded latent infection membrane protein 1-induced NF-kappaB signaling downstream of TRAF2
- PMID: 11932422
- PMCID: PMC155061
- DOI: 10.1128/jvi.76.9.4567-4579.2002
The oncogenic protein kinase Tpl-2/Cot contributes to Epstein-Barr virus-encoded latent infection membrane protein 1-induced NF-kappaB signaling downstream of TRAF2
Abstract
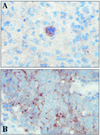
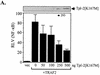
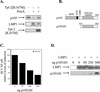
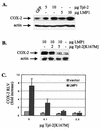
The Epstein-Barr virus-encoded latent infection membrane protein 1 (LMP1) is a pleiotropic protein, the activities of which include effects on cell transformation and phenotype, growth, and survival. The ability of LMP1 to mediate at least some of these phenomena could be attributed to the activation of the transcription factor NF-kappaB. LMP1 promotes NF-kappaB activation through the recruitment of the adapter protein TRAF2 and the formation of a dynamic multiprotein complex that includes the NF-kappaB kinase, the IkappaB kinases, and their downstream targets, IkappaBs and p105. In this study, we have identified the oncogenic kinase Tpl-2/Cot as a novel component of LMP1-induced NF-kappaB signaling. We show that Tpl-2 is expressed in primary biopsies from patients with nasopharyngeal carcinoma and Hodgkin's disease, where LMP1 is also found. Inducible expression of LMP1 promotes the activation of Tpl-2, and a catalytically inactive Tpl-2 mutant suppresses LMP1-induced NF-kappaB signaling. In colocalization and coimmunoprecipitation experiments, Tpl-2 and TRAF2 were found to interact with Tpl-2 functioning downstream of TRAF2. Consistent with this observation, catalytically inactive Tpl-2 also blocked CD40-mediated NF-kappaB activation, which largely depends on TRAF2. The ability of Tpl-2 to influence LMP1-induced NF-kappaB occurs through modulation of both IkappaBalpha and p105 functions. Furthermore, Tpl-2 was found to influence the expression of angiogenic mediators, such as COX-2 in LMP1-transfected cells. These data identify Tpl-2 as a component of LMP1 signaling downstream of TRAF2 and as a modulator of LMP1-mediated effects.
Figures







References
-
- Belich, M. P., A. Salmeron, L. H. Johnston, and S. C. Ley. 1999. Tpl-2 kinase regulates the proteolysis of the NF-κB-inhibitory protein NF-κB1 p105. Nature 397:363-368. - PubMed
-
- Cahir-McFarland, E. D., K. M. Izumi, and G. Mosialos. 1999. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-κB. Oncogene 18:6959-6964. - PubMed
-
- Cammarano, M. S., and A. Minden. 2001. Dbl and the Rho GTPases activate NF-κB by IκB kinase (IKK)-dependent and IKK-independent pathways. J. Biol. Chem. 276:25876-25882. - PubMed
-
- Ceci, J. D., C. P. Patriotis, C. Tsatsanis, A. M. Makris, R. Kovatch, D. A. Swing, N. A. Jenkins, P. N. Tsichlis, and N. G. Copeland. 1997. Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes Dev. 11:688-700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

