Genomic instability in mice lacking histone H2AX
- PMID: 11934988
- PMCID: PMC4721576
- DOI: 10.1126/science.1069398
Genomic instability in mice lacking histone H2AX
Abstract
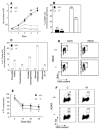
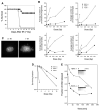
Higher order chromatin structure presents a barrier to the recognition and repair of DNA damage. Double-strand breaks (DSBs) induce histone H2AX phosphorylation, which is associated with the recruitment of repair factors to damaged DNA. To help clarify the physiological role of H2AX, we targeted H2AX in mice. Although H2AX is not essential for irradiation-induced cell-cycle checkpoints, H2AX-/- mice were radiation sensitive, growth retarded, and immune deficient, and mutant males were infertile. These pleiotropic phenotypes were associated with chromosomal instability, repair defects, and impaired recruitment of Nbs1, 53bp1, and Brca1, but not Rad51, to irradiation-induced foci. Thus, H2AX is critical for facilitating the assembly of specific DNA-repair complexes on damaged DNA.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

