Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18
- PMID: 11937493
- PMCID: PMC186326
- DOI: 10.1101/gad.965602
Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18
Abstract
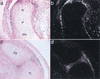
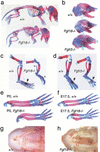
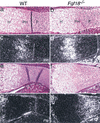
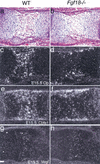
Gain of function mutations in fibroblast growth factor (FGF) receptors cause chondrodysplasia and craniosynostosis syndromes. The ligands interacting with FGF receptors (FGFRs) in developing bone have remained elusive, and the mechanisms by which FGF signaling regulates endochondral, periosteal, and intramembranous bone growth are not known. Here we show that Fgf18 is expressed in the perichondrium and that mice homozygous for a targeted disruption of Fgf18 exhibit a growth plate phenotype similar to that observed in mice lacking Fgfr3 and an ossification defect at sites that express Fgfr2. Mice lacking either Fgf18 or Fgfr3 exhibited expanded zones of proliferating and hypertrophic chondrocytes and increased chondrocyte proliferation, differentiation, and Indian hedgehog signaling. These data suggest that FGF18 acts as a physiological ligand for FGFR3. In addition, mice lacking Fgf18 display delayed ossification and decreased expression of osteogenic markers, phenotypes not seen in mice lacking Fgfr3. These data demonstrate that FGF18 signals through another FGFR to regulate osteoblast growth. Signaling to multiple FGFRs positions FGF18 to coordinate chondrogenesis in the growth plate with osteogenesis in cortical and trabecular bone.
Figures



References
-
- Alvarez J, Horton J, Sohn P, Serra R. The perichondrium plays an important role in mediating the effects of TGF-β1 on endochondral bone formation. Dev Dyn. 2001;221:311–321. - PubMed
-
- Caplan AI, Pechak DG. The cellular and molecular embryology of bone formation. In: Peck WA, editor. Bone and mineral research. New York, NY: Elsevier Science Publishers; 1987. pp. 117–183.
-
- Chen L, Li C, Qiao W, Xu X, Deng C. A Ser(365)→Cys mutation of fibroblast growth factor receptor 3 in mouse downregulates Ihh/PTHrP signals and causes severe achondroplasia. Hum Mol Genet. 2001;10:457–465. - PubMed
-
- Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996;87:553–563. - PubMed
-
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
