Carboxyl-terminal transactivation activity of hypoxia-inducible factor 1 alpha is governed by a von Hippel-Lindau protein-independent, hydroxylation-regulated association with p300/CBP
- PMID: 11940656
- PMCID: PMC133771
- DOI: 10.1128/MCB.22.9.2984-2992.2002
Carboxyl-terminal transactivation activity of hypoxia-inducible factor 1 alpha is governed by a von Hippel-Lindau protein-independent, hydroxylation-regulated association with p300/CBP
Abstract
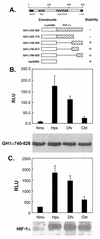
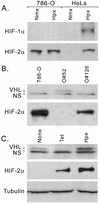
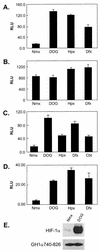
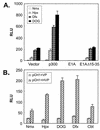
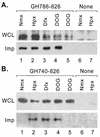
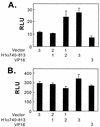
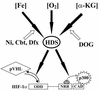
Hypoxia-inducible factor 1 complex (HIF-1) plays a pivotal role in oxygen homeostasis and adaptation to hypoxia. Its function is controlled by both the protein stability and the transactivation activity of its alpha subunit, HIF-1 alpha. Hydroxylation of at least two prolyl residues in the oxygen-dependent degradation domain of HIF-1 alpha regulates its interaction with the von Hippel-Lindau protein (VHL) that targets HIF-1 alpha for ubiquitination and proteasomal degradation. Several prolyl hydroxylases have been found to specifically hydroxylate HIF-1 alpha. In this report, we investigated possible roles of VHL and hydroxylases in the regulation of the transactivation activity of the C-terminal activating domain (CAD) of HIF-1 alpha. We demonstrate that regulation of the transactivation activity of HIF-1 alpha CAD also involves hydroxylase activity but does not require functional VHL. In addition, stimulation of the CAD activity by a hydroxylase inhibitor, hypoxia, and desferrioxamine was severely blocked by the adenoviral oncoprotein E1A but not by an E1A mutant defective in targeting p300/CBP. We further demonstrate that a hydroxylase inhibitor, hypoxia, and desferrioxamine promote the functional and physical interaction between HIF-1 alpha CAD and p300/CBP in vivo. Taken together, our data provide evidence that hypoxia-regulated stabilization and transcriptional stimulation of HIF-1 alpha function are regulated through partially overlapping but distinguishable pathways.
Figures


References
-
- Avantaggiati, M. L., V. Ogryzko, K. Gardner, A. Giordano, A. S. Levine, and K. Kelly. 1997. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89:1175-1184. - PubMed
-
- Beck, I., S. Ramirez, R. Weinmann, and J. Caro. 1991. Enhancer element at the 3′ flanking region controls transcriptional response to hypoxia in the human erythropoietin gene. J. Biol. Chem. 266:15563-15566. - PubMed
-
- Bruick, R. K., and S. L. McKnight. 2001. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337-1340. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
