Role of beta-catenin/T-cell factor-regulated genes in ovarian endometrioid adenocarcinomas
- PMID: 11943708
- PMCID: PMC1867221
- DOI: 10.1016/s0002-9440(10)62550-3
Role of beta-catenin/T-cell factor-regulated genes in ovarian endometrioid adenocarcinomas
Abstract
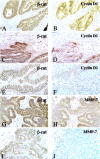
In various cancers, inactivating mutations in the adenomatous polyposis coli or Axin tumor suppressor proteins or activating mutations in beta-catenin's amino-terminal domain elevate beta-catenin levels, resulting in marked effects on T-cell factor (TCF)-regulated transcription. Several candidate beta-catenin/TCF-regulated genes in cancer have been proposed. Expression of a few of these genes has been studied in primary human cancers, but most studies have focused on colon cancers and not on other cancer types that harbor mutational defects in adenomatous polyposis coli, AXIN, or beta-catenin. Mutations leading to beta-catenin deregulation are found in nearly half of ovarian endometrioid adenocarcinomas (OEAs). We report here on the expression of 6 candidate beta-catenin/TCF-regulated genes in a panel of 44 primary OEAs, more than a third of which carry demonstrable defects in beta-catenin regulation. Using quantitative assays of gene expression, we found significantly elevated expression of the MMP-7, CCND1 (Cyclin D1), CX43 (Connexin 43), PPAR-delta, and ITF2 genes in OEAs with deregulated beta-catenin. This correlation was not observed for c-myc, another putative beta-catenin/TCF-regulated gene. Immunohistochemical studies confirmed that overexpression of cyclin D1 and MMP-7 was highly associated with nuclear accumulation of beta-catenin and mutational defects of the Wnt/beta-catenin/TCF-signaling pathway. Our findings indicate cyclin D1, MMP-7, connexin 43, PPAR-delta, and ITF-2, likely play important roles in the pathogenesis of those OEAs that manifest defects in beta-catenin regulation.
Figures


References
-
- Cadigan KM, Nusse R: Wnt signaling: a common theme in animal development. Genes Dev 1997, 11:3286-3305 - PubMed
-
- Polakis P: Wnt signaling and cancer. Genes Dev 2000, 14:1837-1851 - PubMed
-
- Peifer M, Polakis P: Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 2000, 287:1606-1609 - PubMed
-
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 1997, 275:1787-1790 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

