Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-alpha and prostaglandin E(2)
- PMID: 11943733
- PMCID: PMC1867199
- DOI: 10.1016/s0002-9440(10)62575-8
Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-alpha and prostaglandin E(2)
Abstract
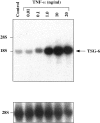
Immediately before parturition the cervix undergoes striking changes in structure (ripening) that facilitate dilatation and effacement. Cervical ripening shares many features in common with inflammation-associated tissue remodeling, making it a valuable process to explore with respect to the biochemical events in extracellular matrix restructuring. Cervical ripening can be pharmacologically induced with prostaglandin E(2) (PGE(2)). Among the biochemical changes in the cervix at parturition is a marked increase in the hyaluronic acid (HA) content. HA and HA-binding proteins have been implicated in tissue hydration, release of collagenase, and leukocyte migration, but their roles in cervical ripening have not been explored. In the present study we examined the ability of PGE(2) to induce expression of the HA-binding protein, tumor necrosis factor-stimulated gene (TSG)-6, in human cervical smooth muscle cells (hCSMCs) and compared the PGE(2) response to that of tumor necrosis factor-alpha (TNF-alpha), an established inducer of TSG-6. TNF-alpha stimulated TSG-6 mRNA accumulation in a dose- and time-dependent manner, with the maximal response observed at 10 ng/ml after 6 hours of incubation. PGE(2) stimulated TSG-6 mRNA expression, but the magnitude of response was substantially less than that produced by TNF-alpha, and it was maximal only after 24 hours of incubation. Quantitative real-time polymerase chain reaction was performed to assess the induction of TSG-6 mRNA and nascent transcripts at 24 hours of treatment. Induction of TSG-6 mRNA and nascent transcripts in response to 10 micromol/L of PGE(2) was 5.7-fold and 6.3-fold greater than control values, respectively, whereas TNF-alpha (10 ng/ml) induced TSG-6 mRNA and nascent transcripts by 80-fold and 134-fold, respectively. TNF-alpha and PGE(2) stimulated secretion of TSG-6 into the culture medium as detected by Western blotting. The effects of PGE(2) on secretion of TSG-6 were delayed compared to TNF-alpha. A 1.3-kb fragment of the human TSG-6 proximal promoter drove luciferase expression in transfected hCSMCs. PGE(2) increased TSG-6 promoter activity 1.75-fold. Paradoxically, TNF-alpha reduced TSG-6 promoter activity by 50%. We conclude that hCSMCs express the hyaladherin TSG-6; that TSG-6 expression in these cells is regulated by PGE(2) as well as proinflammatory cytokines; responses of hCSMCs to TNF-alpha and PGE(2) are distinct in terms of magnitude and the time course; and PGE(2) and TNF-alpha exert different effects on the TSG-6 proximal promoter.
Figures








References
-
- Laurent TC, Fraser JR: Hyaluronan. FASEB J 1992, 6:2397-2404 - PubMed
-
- Comper WD, Laurent TC: Physiological function of connective tissue polysaccharides. Physiol Rev 1978, 58:255-315 - PubMed
-
- Camenisch TD, McDonald JA: Hyaluronan: is bigger better? Am J Respir Cell Mol Biol 2000, 23:431-433 - PubMed
-
- Lee JY, Spicer AP: Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol 2000, 12:581-586 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

