ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels
- PMID: 11943809
- PMCID: PMC6757521
- DOI: 10.1523/JNEUROSCI.22-08-03061.2002
ADP and AMP induce interleukin-1beta release from microglial cells through activation of ATP-primed P2X7 receptor channels
Abstract
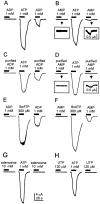
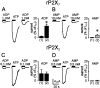
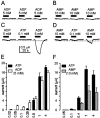
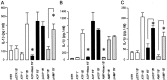
P2X(7) is a subtype of ATP-gated channels that is highly expressed in astrocytes, microglia, and other immune cells. Activation of P2X(7) purinoceptors by ATP or 3'-O-(4-benzoyl)-benzoyl ATP (BzATP) induces the formation of cytolytic pores and provokes release of interleukin-1beta from immune cells. We investigated the actions of other endogenous nucleotides on recombinant and microglial P2X(7) receptors using electrophysiology, fluorescence imaging, and interleukin-1beta release measurement. We found that initial application of ADP or AMP to Xenopus oocytes expressing P2X(7) receptors was ineffective. However, when ADP and AMP, but not UTP or adenosine, were applied after a brief exposure to ATP or BzATP, they activated P2X(7) receptors in a dose-dependent manner. Moreover, responses to ADP and AMP were also elicited after exposure to low concentrations of ATP and were recorded several minutes after removal of ATP from the extracellular medium. Whole-cell recordings from mouse microglial cells showed that significant responses to ADP and AMP were elicited only after ATP application. YO-PRO-1 dye uptake imaging revealed that, unlike ATP, prolonged application of ADP or AMP did not cause an opening of large cytolytic pores in mouse microglial cells. Finally, ADP and AMP stimulated the release of interleukin-1beta from ATP-primed mouse and human microglial cells. We conclude that selective sensitization of P2X(7) receptors to ADP and AMP requires priming with ATP. This novel property of P2X(7) leads to activation by ATP metabolites and proinflammatory cytokine release from microglia without cytotoxicity.
Figures




References
-
- Aloisi F, De Simone R, Columba-Cabezas S, Levi G. Opposite effects of interferon-γ and prostaglandin E2 on tumor necrosis factor and interleukin-10 production in microglia: a regulatory loop controlling microglia pro- and anti-inflammatory activities. J Neurosci Res. 1999;56:571–580. - PubMed
-
- Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G, Di Virgilio F. Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem. 1999;274:33206–33208. - PubMed
-
- Boué-Grabot E, Archambault V, Séguéla P. A protein kinase C site highly conserved in P2X subunits controls the desensitization kinetics of P2X(2) ATP-gated channels. J Biol Chem. 2000;275:10190–10195. - PubMed
-
- Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, Herren S, Gretener D, Grahames C, Kaur R, Kosco-Vilbois MH, Humphrey PP. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–3528. - PubMed
-
- Buttini M, Sauter A, Boddeke HWGM. Induction of interleukin-1 beta messenger RNA after focal cerebral ischaemia in the rat. Mol Brain Res. 1994;23:126–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
