Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia
- PMID: 11943818
- PMCID: PMC6757532
- DOI: 10.1523/JNEUROSCI.22-08-03161.2002
Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia
Abstract
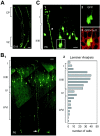
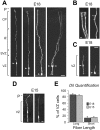
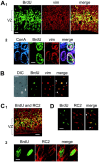
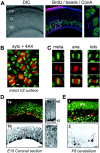
The embryonic ventricular zone (VZ) of the cerebral cortex contains migrating neurons, radial glial cells, and a large population of cycling progenitor cells that generate newborn neurons. The latter two cell classes have been assumed for some time to be distinct in both function and anatomy, but the cellular anatomy of the progenitor cell type has remained poorly defined. Several recent reports have raised doubts about the distinction between radial glial and precursor cells by demonstrating that radial glial cells are themselves neuronal progenitor cells (Malatesta et al., 2000; Hartfuss et al., 2001; Miyata et al., 2001; Noctor et al., 2001). This discovery raises the possibility that radial glia and the population of VZ progenitor cells may be one anatomical and functional cell class. Such a hypothesis predicts that throughout neurogenesis almost all mitotically active VZ cells and a substantial percentage of VZ cells overall are radial glia. We have therefore used various anatomical, immunohistochemical, and electrophysiological techniques to test these predictions. Our data demonstrate that the majority of VZ cells, and nearly all mitotically active VZ cells during neurogenesis, both have radial glial morphology and express radial glial markers. In addition, intracellular dye filling of electrophysiologically characterized progenitor cells in the VZ demonstrates that these cells have the morphology of radial glia. Because the vast majority cycling cells in the cortical VZ have characteristics of radial glia, the radial glial precursor cell may be responsible for both the production of newborn neurons and the guidance of daughter neurons to their destinations in the developing cortex.
Figures


References
-
- Altman J, Bayer SA. Vertical compartmentation and cellular transformations in the germinal matrices of the embryonic rat cerebral cortex. Exp Neurol. 1990;107:23–35. - PubMed
-
- Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264:159–170. - PubMed
-
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. - PubMed
-
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. - PubMed
-
- Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
