Somatosensory cortex dominated by the representation of teeth in the naked mole-rat brain
- PMID: 11943853
- PMCID: PMC122833
- DOI: 10.1073/pnas.072097999
Somatosensory cortex dominated by the representation of teeth in the naked mole-rat brain
Abstract
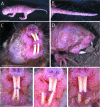
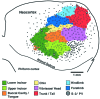
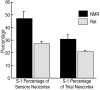
We investigated naked mole-rat somatosensory cortex to determine how brain areas are modified in mammals with unusual and extreme sensory specializations. Naked mole-rats (Heterocephalus glaber) have numerous anatomical specializations for a subterranean existence, including rows of sensory hairs along the body and tail, reduced eyes, and ears sensitive to low frequencies. However, chief among their adaptations are behaviorally important, enlarged incisors permanently exterior to the oral cavity that are used for digging, object manipulation, social interactions, and feeding. Here we report an extraordinary brain organization where nearly one-third (31%) of primary somatosensory cortex is devoted to the representations of the upper and lower incisors. In addition, somatosensory cortex is greatly enlarged (as a proportion of total neocortical area) compared with closely related laboratory rats. Finally, somatosensory cortex in naked mole-rats encompasses virtually all of the neocortex normally devoted to vision. These findings indicate that major cortical remodeling has occurred in naked mole-rats, paralleling the anatomical and behavioral specializations related to fossorial life.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

