A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles
- PMID: 11950943
- PMCID: PMC102273
- DOI: 10.1091/mbc.01-11-0544
A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles
Abstract
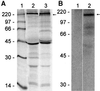
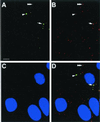
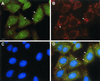
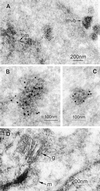
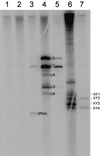
A novel human cellular structure has been identified that contains a unique autoimmune antigen and multiple messenger RNAs. This complex was discovered using an autoimmune serum from a patient with motor and sensory neuropathy and contains a protein of 182 kDa. The gene and cDNA encoding the protein indicated an open reading frame with glycine-tryptophan (GW) repeats and a single RNA recognition motif. Both the patient's serum and a rabbit serum raised against the recombinant GW protein costained discrete cytoplasmic speckles designated as GW bodies (GWBs) that do not overlap with the Golgi complex, endosomes, lysosomes, or peroxisomes. The mRNAs associated with GW182 represent a clustered set of transcripts that are presumed to reside within the GW complexes. We propose that the GW ribonucleoprotein complex is involved in the posttranscriptional regulation of gene expression by sequestering a specific subset of gene transcripts involved in cell growth and homeostasis.
Figures






References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Antic D, Keene JD. Messenger ribonucleoprotein complexes containing human ELAV proteins: interactions with cytoskeleton and translational apparatus. J Cell Sci. 1998;111:183–197. - PubMed
-
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

