Biochemical and molecular inhibition of plastidial carbonic anhydrase reduces the incorporation of acetate into lipids in cotton embryos and tobacco cell suspensions and leaves
- PMID: 11950990
- PMCID: PMC154269
- DOI: 10.1104/pp.010879
Biochemical and molecular inhibition of plastidial carbonic anhydrase reduces the incorporation of acetate into lipids in cotton embryos and tobacco cell suspensions and leaves
Abstract
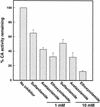
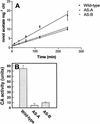
Two cDNAs encoding functional carbonic anhydrase (CA) enzymes were recently isolated from a non-photosynthetic, cotyledon library of cotton (Gossypium hirsutum) seedlings with putative plastid-targeting sequences (GenBank accession nos. AF132854 and AF132855). Relative CA transcript abundance and enzyme activity increased 9 and 15 times, respectively, in cotton embryos during the maximum period of reserve oil accumulation. Specific sulfonamide inhibitors of CA activity significantly reduced the rate of [(14)C]acetate incorporation into total lipids in cotton embryos in vivo, and in embryo plastids in vitro, suggesting a role for CA in plastid lipid biosynthesis. CA inhibitors did not affect acetyl-coenzyme A carboxylase activity or total storage protein synthesis. Similar results were obtained for two other plant systems: cell suspensions (and isolated plastids therefrom) of tobacco (Nicotiana tabacum), and chloroplasts isolated from leaves of transgenic CA antisense-suppressed tobacco plants (5% of wild-type CA activity). In addition, tobacco cell suspensions treated with the CA inhibitor ethoxyzolamide showed a substantial loss of CO(2) compared with controls. The rate of [(14)C]acetate incorporation into lipid in cell suspensions was reduced by limiting external [CO(2)] (scrubbed air), and this rate was further reduced in the presence of ethoxyzolamide. Together, these results indicate that a reduction of CA activity (biochemical or molecular inhibition) impacts the rate of plant lipid biosynthesis from acetate, perhaps by impairing the ability of CA to efficiently "trap" inorganic carbon inside plastids for utilization by acetyl-coenzyme A carboxylase and the fatty acid synthesis machinery.
Figures




References
-
- Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. - PubMed
-
- Badger MR, Price GD. The CO2 concentrating mechanism in cyanobacteria and green algae. Physiol Plant. 1992;84:606–615.
-
- Badger MR, Price GD. The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:369–392.
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;12:248–254. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

