Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation
- PMID: 11953316
- PMCID: PMC125972
- DOI: 10.1093/emboj/21.8.1967
Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation
Abstract
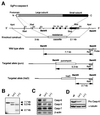
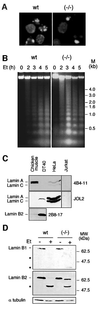
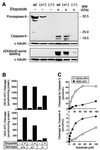
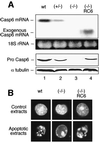
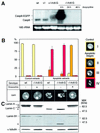
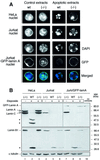
To study the role of caspase-6 during nuclear disassembly, we generated a chicken DT40 cell line in which both alleles of the caspase-6 gene were disrupted. No obvious morphological differences were observed in the apoptotic process in caspase-6- deficient cells compared with the wild type. However, examination of apoptosis in a cell-free system revealed a block in chromatin condensation and apoptotic body formation when nuclei from HeLa cells expressing lamin A or lamin A-transfected Jurkat cells were incubated in caspase-6-deficient apoptotic extracts. Transfection of exogenous caspase-6 into the clone reversed this phenotype. Lamins A and C, which are caspase-6-only substrates, were cleaved by the wild-type and heterozygous apoptotic extracts but not by the extracts lacking caspase-6. Furthermore, the caspase-6 inhibitor z-VEID-fmk mimicked the effects of caspase-6 deficiency and prevented the cleavage of lamin A. Taken together, these observations indicate that caspase-6 activity is essential for lamin A cleavage and that when lamin A is present it must be cleaved in order for the chromosomal DNA to undergo complete condensation during apoptotic execution.
Figures

References
-
- Allsopp T.E., McLuckie,J., Kerr,L.E., Macleod,M., Sharkey,J. and Kelly,J.S. (2000) Caspase 6 activity initiates caspase 3 activation in cerebellar granule cell apoptosis. Cell Death Differ., 7, 984–993. - PubMed
-
- Benavente R., Krohne,G. and Franke,W.W. (1985) Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell, 41, 177–190. - PubMed
-
- Bonne G. et al. (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery–Dreifuss muscular dystrophy. Nature Genet., 21, 285–288. - PubMed
-
- Budihardjo I., Oliver,H., Lutter,M., Luo,X. and Wang,X. (1999) Biochemical pathways of caspase activation during apoptosis. Annu. Rev. Cell Dev. Biol., 15, 269–290. - PubMed
-
- Buerstedde J.-M. and Takeda,S. (1991) Increased ratio of targeted to random integration after transfection of chicken B cell lines. Cell, 67, 179–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

