The active site of the DNA repair endonuclease XPF-ERCC1 forms a highly conserved nuclease motif
- PMID: 11953324
- PMCID: PMC125967
- DOI: 10.1093/emboj/21.8.2045
The active site of the DNA repair endonuclease XPF-ERCC1 forms a highly conserved nuclease motif
Abstract
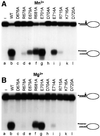
XPF-ERCC1 is a structure-specific endonuclease involved in nucleotide excision repair, interstrand crosslink repair and homologous recombination. So far, it has not been shown experimentally which subunit of the heterodimer harbors the nuclease activity and which amino acids contribute to catalysis. We used an affinity cleavage assay and located the active site to amino acids 670-740 of XPF. Point mutations generated in this region were analyzed for their role in nuclease activity, metal coordination and DNA binding. Several acidic and basic residues turned out to be required for nuclease activity, but not DNA binding. The separation of substrate binding and catalysis by XPF-ERCC1 will be invaluable in studying the role of this protein in various DNA repair processes. Alignment of the active site region of XPF with proteins belonging to the Mus81 family and a putative archaeal RNA helicase family reveals that seven of the residues of XPF involved in nuclease activity are absolutely conserved in the three protein families, indicating that they share a common nuclease motif.
Figures
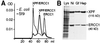




References
-
- Aboussekhra A. et al. (1995) Mammalian DNA nucleotide excision repair reconstituted with purified protein components. Cell, 80, 859–868. - PubMed
-
- Bardwell A.J., Bardwell,L., Tomkinson,A.E. and Friedberg,E.C. (1994) Specific cleavage of model recombination and repair intermediates by the yeast Rad1–Rad10 DNA endonuclease. Science, 265, 2082–2085. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

