Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo
- PMID: 11953397
- PMCID: PMC127896
- DOI: 10.1128/IAI.70.5.2566-2575.2002
Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo
Abstract
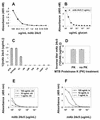
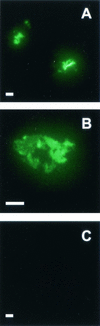
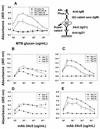
The outermost layer of Mycobacterium tuberculosis is composed primarily of two polysaccharides, glucan (GC) and arabinomannan. To analyze the surface polysaccharide composition of M. tuberculosis, we generated a monoclonal antibody (MAb) that binds M. tuberculosis GC and is known as MAb 24c5. Immunofluorescence and whole-mount immunoelectron microscopy indicated that GC is on the outermost portion of the bacteria. M. tuberculosis strains Erdman and CDC 1551 were analyzed for their ability to bind MAb 24c5 after in vitro growth in media with and without the detergent Tween 80. MAb 24c5 bound to Erdman and CDC 1551 at all culture times with only slightly greater apparent affinity after extended culture in the absence of Tween 80, indicating that a stable amount of GC polysaccharide antigen is associated with the cell surface of M. tuberculosis. An enzyme-linked immunosorbent assay indicated that GC is antigenically similar to glycogen, and the amount of GC antigen increased in the media of M. tuberculosis cultures grown either with or without the detergent Tween 80. Other nontuberculosis mycobacteria have antigenically similar GCs on their surfaces after in vitro growth. Inoculation of mice with live bacilli but not inoculation with dead bacilli elicited a strong antibody response to GC consistent with production of this antigen in vivo. Our results provide a more comprehensive picture of the M. tuberculosis cell envelope and the conditions that allow expression of M. tuberculosis GC.
Figures
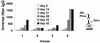



References
-
- Bloom, B. R., and C. J. Murray. 1992. Tuberculosis: commentary on a reemergent killer. Science 257:1055-1064. - PubMed
-
- Brennan, P. J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29-63. - PubMed
-
- Casadevall, A., J. Mukherjee, and M. D. Scharff. 1992. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods 154:27-35. - PubMed
-
- Chatterjee, D. 1997. The mycobacterial cell wall: structure, biosynthesis and sites of drug action. Curr. Opin. Chem. Biol. 1:579-588. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI033142/AI/NIAID NIH HHS/United States
- 1K08AI01691/AI/NIAID NIH HHS/United States
- AI33774/AI/NIAID NIH HHS/United States
- R01 AI033774/AI/NIAID NIH HHS/United States
- R01 HL059842/HL/NHLBI NIH HHS/United States
- T32 AI007501/AI/NIAID NIH HHS/United States
- CA 13330/CA/NCI NIH HHS/United States
- AI33142/AI/NIAID NIH HHS/United States
- HL59842/HL/NHLBI NIH HHS/United States
- N01-AI-75320/AI/NIAID NIH HHS/United States
- K08 AI001691/AI/NIAID NIH HHS/United States
- R37 AI033142/AI/NIAID NIH HHS/United States
- P30 CA013330/CA/NCI NIH HHS/United States
- 5T32AI07501/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

